Folic acid
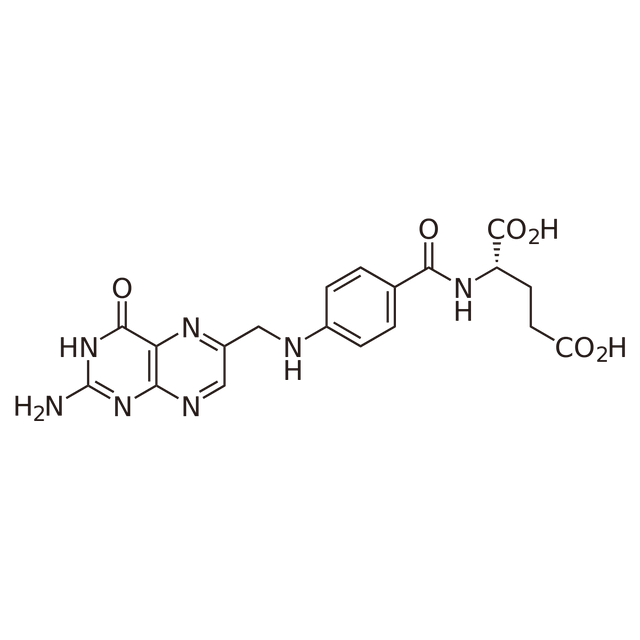
Folic acid

| Clinical data | |
|---|---|
| Pronunciation | |
| Synonyms | FA,N-(4-{[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid, pteroyl-L-glutamic acid, vitamin B,[1]vitamin B,[2]] vitamin M,[3]folacin, pteroyl-L-glutamate |
| AHFS/Drugs.com | |
| MedlinePlus | |
| Pregnancy category | |
| Routes of administration | By mouth, IM, IV, sub-Q |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 50–100%[4] |
| Metabolism | Liver[4] |
| Excretion | Urine[4] |
| Identifiers | |
| CAS Number | |
| PubChem | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard | |
| ECHA InfoCard | 100.000.381[154] |
| Chemical and physical data | |
| Formula | |
| Molar mass | g·mol |
| 3D model (JSmol) | |
| Density | 1.6±0.1[6]g/cm |
| Melting point | 250 °C (482 °F) (decomposition) |
| Solubility in water | 1.6 mg/L (25 °C) mg/mL (20 °C) |
Folate, distinct forms of which are known as folic acid, folacin, and vitamin B9,[7] is one of the B vitamins.[4] Folate is essential for the body to make DNA, RNA, and metabolise amino acids, which are required for cell division.[8][9] As humans cannot make folate, it is required from the diet, making it an essential vitamin.[10] It occurs naturally in many foods.[7][8] The recommended adult daily intake of folate in the U.S. is 400 micrograms from foods or dietary supplements.[8]
Folate in the form of folic acid is used to treat anemia caused by folate deficiency.[4] Folic acid is also used as a supplement by women during pregnancy to reduce the risk of neural tube defects (NTDs) in the baby.[4][11] Low levels in early pregnancy are believed to be the cause of more than half of babies born with NTDs.[8] More than 80 countries use fortification of certain foods with folic acid as a measure to decrease the rate of NTDs.[12] Long-term supplementation is also associated with small reductions in the risk of stroke and cardiovascular disease.[13] No common side effects are known.[4] There are concerns that large amounts of folic acid might hide vitamin B12 deficiency.[8]
Not consuming enough folate can lead to folate deficiency.[8] This may result in a type of anemia in which low numbers of large red blood cells occur.[8] Symptoms may include feeling tired, heart palpitations, shortness of breath, open sores on the tongue, and changes in the color of the skin or hair.[8] Folate deficiency in children may develop within a month of poor dietary intake.[14] In adults, normal total body folate is between 10 and 30 mg with blood levels of greater than 7 nmol/L (3 ng/mL).[8]
Folic acid was discovered between 1931 and 1943.[15] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[16] The wholesale cost of supplements in the developing world is between US$0.001 and 0.005 per dose as of 2014.[17] The term "folic" is from the Latin word folium (which means leaf) because it was found in dark-green leafy vegetables.[18] In 2016, it was the 96th most prescribed medication in the United States, with more than 8 million prescriptions.[19]
| Clinical data | |
|---|---|
| Pronunciation | |
| Synonyms | FA,N-(4-{[(2-amino-4-oxo-1,4-dihydropteridin-6-yl)methyl]amino}benzoyl)-L-glutamic acid, pteroyl-L-glutamic acid, vitamin B,[1]vitamin B,[2]] vitamin M,[3]folacin, pteroyl-L-glutamate |
| AHFS/Drugs.com | |
| MedlinePlus | |
| Pregnancy category | |
| Routes of administration | By mouth, IM, IV, sub-Q |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 50–100%[4] |
| Metabolism | Liver[4] |
| Excretion | Urine[4] |
| Identifiers | |
| CAS Number | |
| PubChem | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard | |
| ECHA InfoCard | 100.000.381[154] |
| Chemical and physical data | |
| Formula | |
| Molar mass | g·mol |
| 3D model (JSmol) | |
| Density | 1.6±0.1[6]g/cm |
| Melting point | 250 °C (482 °F) (decomposition) |
| Solubility in water | 1.6 mg/L (25 °C) mg/mL (20 °C) |
Definition

Chemical structure of the folate family
"Folate" refers to the many forms of folic acid and its related compounds, including tetrahydrofolic acid (the active form), methyltetrahydrofolate (the primary form found in blood), methenyltetrahydrofolate, folinic acid, and folacin.[7][20][21][22] Other names include vitamin B9,[1] vitamin Bc,[2] and vitamin M.[3]
Chemically, folates consist of three distinct chemical moieties linked together.
A pterin (2-amino-4-hydroxy-pteridine) heterocyclic ring is linked by a methylene bridge to a p-aminobenzoyl group that in turn is bonded through a amide linkage to either glutamic acid or poly-glutamate. One-carbon units in a variety of oxidation states may be attached to the N5 nitrogen atom of the pteridine ring and/or the N10 nitrogen atom of the p-aminobenzoyl group.[23]
Health effects
Folate is especially important during periods of frequent cell division and growth, such as infancy and pregnancy.
Folate deficiency hinders DNA synthesis and cell division, affecting hematopoietic cells and neoplasms the most because of their greater frequency of cell division. RNA transcription and subsequent protein synthesis are less affected by folate deficiency, as the mRNA can be recycled and used again (as opposed to DNA synthesis, where a new genomic copy must be created).
Folate deficiency
Folate deficiency can be caused by unhealthy diets that do not include enough vegetables and other folate-rich foods; diseases in which folates are not well absorbed in the digestive system (such as Crohn's disease or celiac disease); some genetic disorders that affect levels of folate; and certain medicines (such as phenytoin, sulfasalazine, or trimethoprim-sulfamethoxazole).[24] Folate deficiency is accelerated by alcohol consumption, possibly by interference with folate transport.[25]
Folate deficiency may lead to glossitis, diarrhea, depression, confusion, anemia, and fetal neural tube and brain defects.[26] Other symptoms include fatigue, gray hair, mouth sores, poor growth, and swollen tongue.[24] Folate deficiency is diagnosed by analyzing a Complete blood count (CBC) and plasma vitamin B12 and folate levels. A serum folate of 3 μg/L or lower indicates deficiency.[26] Serum folate level reflects folate status, but erythrocyte folate level better reflects tissue stores after intake. An erythrocyte folate level of 140 μg/L or lower indicates inadequate folate status. Serum folate reacts more rapidly to folate intake than erythrocyte folate.[27]
Since folate deficiency limits cell division, erythropoiesis (production of red blood cells) is hindered. This leads to megaloblastic anemia, which is characterized by large, immature red blood cells. This pathology results from persistently thwarted attempts at normal DNA replication, DNA repair, and cell division, and produces abnormally large red cells called megaloblasts (and hypersegmented neutrophils) with abundant cytoplasm capable of RNA and protein synthesis, but with clumping and fragmentation of nuclear chromatin. Some of these large cells, although immature (reticulocytes), are released early from the marrow in an attempt to compensate for the anemia.[28] Both adults and children need folate to make normal red and white blood cells and prevent anemia, which causes fatigue, weakness, and inability to concentrate.[29][30]
Increased homocysteine levels suggest tissue folate deficiency, but homocysteine is also affected by vitamin B12 and vitamin B6, renal function, and genetics.
One way to differentiate between folate deficiency and vitamin B12 deficiency is by testing for methylmalonic acid (MMA) levels. Normal MMA levels indicate folate deficiency and elevated MMA levels indicate vitamin B12 deficiency.[26] Folate deficiency is treated with supplemental oral folic acid of 400 to 1000 μg per day. This treatment is very successful in replenishing tissues, even if deficiency was caused by malabsorption. People with megaloblastic anemia need to be tested for vitamin B12 deficiency before treatment with folic acid, because if the person has vitamin B12 deficiency, folic acid supplementation can remove the anemia, but can also worsen neurologic problems.[26] Cobalamin deficiency may lead to folate deficiency, which, in turn, increases homocysteine levels and may result in the development of cardiovascular disease or birth defects.[31]
Birth defects
Deficiency of folate in pregnant women has been implicated in neural tube defects (NTDs), with an estimate of 300,000 cases worldwide prior to the implementation in many countries of mandatory foor fortification.[32] NTDs occur early in pregnancy (first month), therefore women must have abundant folate upon conception and for this reason there is a recommendation that any woman planning to become pregnant consume a folate-containing dietary supplement before and during pregnancy.[33] Compliance with this recommendation is not complete, and many women become pregnant without this being a planned pregnancy, or may not realize that they are pregnant until well into the first trimester, which is the critical period for reducing risk of NTDs. Countries have implemented either mandatory or voluntary food fortification of wheat flour and other grains,[34] or else have no such program and depend on public health and healthcare practitioner advice to women of childbearing age. A meta-analysis of global birth prevalence of spina bifida showed that when mandatory fortification was compared to countries with voluntary fortification or no fortification program, there was a 30% reduction in live births with spina bifida.[35] Some countries reported a greater than 50% reduction.[36] The United States Preventive Services Task Force recommends folic acid as the supplement or fortification ingredient, as forms of folate other than folic acid have not been studied.[21]
A meta-analysis of folate supplementation during pregnancy reported a 28% lower relative risk of newborn congenital heart defects.[37] Prenatal supplementation with folic acid did not appear to reduce the risk of preterm births.[38][39] One systematic review indicated no effect of folic acid on mortality, growth, body composition, respiratory, or cognitive outcomes of children from birth to 9 years old.[40] There was no relation between maternal folic acid supplementation and an increased risk for childhood asthma.[41]
Fertility
Heart disease
One meta-analysis reported that multi-year folic acid supplementation, in amounts in most of the included clinical trials at higher than the UL of 1,000 μg/day, reduced the relative risk of cardiovascular disease by a modest but statistically significant 4%.[13] Two older meta-analyses, which would not have incorporated results from newer clinical trials, reported no changes to the risk of cardiovascular disease.[43][44]
Stroke
The absolute risk of stroke with supplementation decreases from 4.4% to 3.8% (a 10% decrease in relative risk).[13] Two other meta-analyses reported a similar decrease in relative risk.[45][46] Two of these three were limited to people with pre-existing cardiovascular disease or coronary heart disease.[13][45] The beneficial result may be associated with lowering circulating homocysteine concentration, as stratified analysis showed that risk was reduced more when there was a larger decrease in homocysteine.[13][45] The effect was also larger for the studies that were conducted in countries that did not have mandatory grain folic acid fortification.[45][46] The beneficial effect was larger in the subset of trials that used a lower folic acid supplement compared to higher.[45][46]
Cancer
Early after fortification programs were implemented, high intakes were theorized to accelerate the growth of preneoplastic lesions that could lead to cancer, specifically colon cancer.[49] Subsequent meta-analyses of the effects of low versus high dietary folate, elevated serum folate, and supplemental folate in the form of folic acid have reported at times conflicting results.
Comparing low to high dietary folate showed a modest but statistically significant reduced risk of colon cancer.[50] For prostate cancer risk, comparing low to high dietary folate showed no effect,[51][52] but the same two studies reported a significant increased risk for prostate cancer correlating to elevated serum folate.[51][52] Two reviews of trials that involved folic acid dietary supplements reported, respectively, a statistically significant 24% increase in prostate cancer risk[53] and a not significant 17% increase in prostate cancer risk.[54] Supplementation with folic acid at 1,000 to 2,500 μg/day - the amounts used in many of the supplement trials[53][54] - would result in higher concentrations of serum folate than what is achieved from diets high in food-derived folate. The second study reported no significant increase or decrease in total cancer incidence, colorectal cancer, other gastrointestinal cancer, genitourinary cancer, lung cancer or hematological malignancies in people who were consuming folic acid supplements.[54] A third supplementation meta-analysis limited to reporting only on colorectal cancer incidence showed that folic acid treatment was not associated with colorectal cancer risk.[55]
Anti-folate chemotherapy
Folate is important for cells and tissues that divide rapidly.[56] Cancer cells divide rapidly, and drugs that interfere with folate metabolism are used to treat cancer.
The antifolate drug methotrexate is often used to treat cancer because it inhibits the production of the active form of THF from the inactive dihydrofolate (DHF). However, methotrexate can be toxic,[57][58][59] producing side effects, such as inflammation in the digestive tract that make eating normally more difficult. Also, bone marrow depression (inducing leukopenia and thrombocytopenia) and acute kidney and liver failure have been reported.
Folinic acid, under the drug name leucovorin, a form of folate (formyl-THF), can help "rescue" or reverse the toxic effects of methotrexate.[60] Folinic acid is not the same as folic acid. Folic acid supplements have little established role in cancer chemotherapy.[61][62] Cases of severe adverse effects of accidental substitution of folic acid for folinic acid have been reported in people receiving methotrexate cancer chemotherapy. Anyone receiving methotrexate should follow medical advice on the use of folic or folinic acid supplements. The supplement of folinic acid in people undergoing methotrexate treatment is to give cells dividing less rapidly enough folate to maintain normal cell functions. The amount of folate given is depleted by rapidly dividing cells (cancer) quickly, so does not negate the effects of methotrexate.
Neurological
Some evidence links a shortage of folate with clinical depression.[63] Limited evidence from randomized controlled trials showed using folic acid in addition to selective serotonin reuptake inhibitors (SSRIs) may have benefits.[64] Research found a link between depression and low levels of folate.[65][66] Folate may reduce homocysteine levels, which are associated with cognitive functions.[7]
The exact mechanisms involved in the development of schizophrenia and depression are not entirely clear, but the bioactive folate, methyltetrahydrofolate (5-MTHF), a direct target of methyl donors such as S-adenosyl methionine (SAMe), recycles the inactive dihydrobiopterin (BH2) into tetrahydrobiopterin (BH4), the necessary cofactor in various steps of monoamine synthesis, including that of dopamine. BH4 serves a regulatory role in monoamine neurotransmission and is required to mediate the actions of most antidepressants. 5-MTHF also plays both direct & indirect roles in DNA methylation, NO2 synthesis, and one-carbon metabolism.[67]
Age-related macular degeneration
A sub-study of the Women's Antioxidant and Folic Acid Cardiovascular Study published in 2009 reported use of a nutritional supplement containing folic acid at 2,500 μg/day, pyridoxine at 50 mg/day, and vitamin B12 at 1,000 μg/day decreased the risk of developing age-related macular degeneration by 34.7%. The amount of folic acid used in this clinical trial – 2,500 μg/day – was higher than the tolerable upper intake level of 1,000 μg.[68]
Folic acid, B12 and iron
Malaria
Metabolism
The biological activity of folate the body depends upon dihydrofolate reductase action in the liver which converts folate into tetrahydrofolate (THF). This action is rate-limiting in humans leading to elevated blood concentrations of unmetabolized folic acid when consumption from dietary supplements and fortified foods nears or exceeds the U.S. Tolerable Upper Intake Level of 1,000 μg per day.[9][73]
Biosynthesis
Animals including humans cannot synthesize folate and therefore must obtain folate from their diet.
All plants and fungi and certain protozoa, bacteria, and archaea can synthesize folate de novo through variations on the same biosynthetic pathway.[74] The folate molecule is synthesized from pterin pyrophosphate and para-aminobenzoic acid through the action of dihydrofolate synthase. Pterin is in turn derived in a series of enzymatically catalyzed steps from guanosine triphosphate (GTP), while para-aminobenzoic acid (vitamin B10) is a product of the shikimate pathway.[74]
Bioactivation

Biotransformation of folic acid into folinic acids where R = para-aminobenzoate-glutamate.[75]
All of the biological functions of folic acid are performed by tetrahydrofolate (THF) and its methylated derivatives. Hence folic acid must first be reduced to THF. This four electron reduction proceeds in two chemical steps both catalyzed by the same enzyme, dihydrofolate reductase.[75] Folic acid is first reduced to dihydrofolate and then to tetrahydrofolate. Each step consumes one molecule of NADPH (biosynthetically derived from vitamin B3) and produces one molecule of NADP.[9][76] Mechanistically, hydride is transferred from NADPH to the C6 position of the pteridine ring.[77]
A C1 methyl group is added to tetrahydrofolate through the action of serine hydroxymethyltransferase (SHMT) to yield 5,10-methylenetetrahydrofolate (5,10-CH2-THF). This reaction also consumes serine and pyridoxal phosphate (PLP; vitamin B6) and produces glycine and pyridoxal.[75] A second enzyme, methylenetetrahydrofolate dehydrogenase (MTHFD2)[78] oxidizes 5,10-methylenetetrahydrofolate to an iminium cation which in turn is hydrolyzed to produce 5-formy-THF and 10-formyl-THF.[75] This series of reactions using the β-carbon atom of serine as the carbon source provide the largest part of the one-carbon units available to the cell.[79]
Alternative carbon sources include formate which by the catalytic action of formate–tetrahydrofolate ligase add a C1 unit to THF to yield 10-formyl-THF. Glycine, histidine, and sarcosine can also directly contribute to the THF-bound 1C pool.[80]
Drug interference
A number of drugs interfere with the biosynthesis of THF from folic acid.
Among them are the dihydrofolate reductase inhibitors such as trimethoprim, pyrimethamine, and methotrexate; the sulfonamides (competitive inhibitors of 4-aminobenzoic acid in the reactions of dihydropteroate synthetase). Valproic acid, one of the most commonly prescribed anticonvulsants that is also used to treat certain psychological conditions, is a known inhibitor of folic acid, and as such, has been shown to cause neural tube defects and cases of spina bifida and cognitive impairment in the newborn. Because of this considerable risk, those mothers who must continue to use valproic acid or its derivatives during pregnancy to control their condition (as opposed to stopping the drug or switching to another drug or to a lesser dose) should take folic acid supplements under the direction and guidance of their health care providers.
Function
Tetrahydrofolate's main function in metabolism is transporting single-carbon groups (i.e. a methyl group, methylene group, or formyl group). These carbon groups can be transferred to other molecules as part of the modification or biosynthesis of a variety of biological molecules. Folates are essential for the synthesis of DNA, the modification of DNA and RNA, the synthesis of methionine from homocysteine, and various other chemical reactions involved in cellular metabolism.[81] These reactions are collectively known as folate-mediated one-carbon metabolism.[9][82]
DNA synthesis
Folate derivatives participate in the biosynthesis of both purines and pyrimidines.
Formyl folate is required for two of the steps in the biosynthesis of inosine monophosphate, the precursor to GMP and AMP. Methylenetetrahydrofolate donates the C1 center required for the biosynthesis of dTMP (2′-deoxythymidine-5′-phosphate) from dUMP (2′-deoxyuridine-5′-phosphate).
Formyl folate is required for two of the steps in the biosynthesis of inosine monophosphate, the precursor to GMP and AMP. Methylenetetrahydrofolate donates the C1 center required for the biosynthesis of dTMP (2′-deoxythymidine-5′-phosphate) from dUMP (2′-deoxyuridine-5′-phosphate). The conversion is catalyzed by thymidylate synthase.[9]
Vitamin B12 activation

Simplified schematic diagram of the folate methionine cycle[83]
Methyl-THF converts vitamin B12 to methyl-B12 (methylcobalamin). Methyl-B12 converts homocysteine, in a reaction catalyzed by homocysteine methyltransferase, to methionine. A defect in homocysteine methyltransferase or a deficiency of B12 may lead to a so-called "methyl-trap" of THF, in which THF converts to methyl-THF, causing a deficiency in folate.[84] Thus, a deficiency in B12 can cause accumulation of methyl-THF, mimicking folate deficiency
Diet
The United States Department of Agriculture, Agricultural Research Service, maintains a food composition database from which folate content in hundreds of foods can be searched as shown in the table.[85] The Food Fortification Initiative lists all countries in the world that conduct fortification programs,[86] and within each country, what nutrients are added to which foods, and whether those programs are voluntary or mandatory. In the US, mandatory fortification of enriched breads, cereals, flours, corn meal, pastas, rice, and other grain products began in January 1998. As of December 21, 2018, 81 countries required food fortification with one or more vitamins.[34] The most commonly fortified vitamin – as used in 62 countries – is folate; the most commonly fortified food is wheat flour, followed by maize flour and rice. From country to country, added folic acid amounts range from 0.4 to 5.1 μg/100 g, but the great majority are in a more narrow range of 1.5 to 2.5 μg/100 g.[34] Folate naturally found in food is susceptible to destruction from high heat cooking, especially in the presence of acidic foods and sauces. It is soluble in water, and so may be lost from foods boiled in water.[87] For foods that are normally consumed cooked, values in the table are for folate naturally occurring in cooked foods.
Dietary recommendations
Because of the difference in bioavailability between supplemented folic acid and the different forms of folate found in food, the dietary folate equivalent (DFE) system was established.
One DFE is defined as 1 μg of dietary folate.
One μg of folic acid supplement counts as 1.7 μg DFE.
The reason for the difference is that when folic acid is added to food or taken as a dietary supplement with food is at least 85% absorbed, whereas only about 50% of folate naturally present in food is absorbed.[8]
| Age | Infants | Children and adults | Pregnant women | Lactating women | ||||
|---|---|---|---|---|---|---|---|---|
| (AI) | (UL) | (RDA) | (UL) | (RDA) | (UL) | (RDA) | (UL) | |
| 0–6 months | 65 | None set | – | – | – | – | – | – |
| 7–12 months | 80 | None set | – | – | – | – | – | – |
| 1–3 years | – | – | 150 | 300 | – | – | – | – |
| 4–8 years | – | – | 200 | 400 | – | – | – | – |
| 9–13 years | – | – | 300 | 600 | – | – | – | – |
| 14–18 | – | – | 400 | 800 | 600 | 800 | 500 | 800 |
| 19+ | – | – | 400 | 1000 | 600 | 1000 | 500 | 1000 |
The U.S. Institute of Medicine updated Recommended Dietary Allowances (RDAs) and Tolerable upper intake levels (ULs) for folate in 2001. Collectively the EARs, RDAs, AIs, and ULs are referred to as Dietary Reference Intakes (DRIs).[8][26] The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women and men over age 18 the PRI is set at 330 μg/day. PRI for pregnancy is 600 μg/day, for lactation 500 μg/day. For children ages 1–17 years the PRIs increase with age from 120 to 270 μg/day. These values differ somewhat from the U.S. RDAs.[88] The United Kingdom's Dietary Reference Value for folate, set by the Committee on Medical Aspects of Food and Nutrition Policy in 1991, is 200 μg/day for adults.[89]
Safety
The risk of toxicity from folic acid is low, because folate is a water-soluble vitamin and is regularly removed from the body through urine.
One potential issue associated with high doses of folic acid is that it has a masking effect on the diagnosis of pernicious anaemia due to vitamin B12 deficiency, and may even precipitate or exacerbate neuropathy in vitamin B12-deficient individuals. This evidence justified development of a UL for folate.[26] In general, ULs are set for vitamins and minerals when evidence is sufficient. The adult UL of 1,000 μg for folate (and lower for children) refers specifically to folic acid used as a supplement, as no health risks have been associated with high intake of folate from food sources. The EFSA reviewed the safety question and agreed with United States that the UL be set at 1,000 μg.[90] The Japan National Institute of Health and Nutrition set the adult UL at 1,300 or 1,400 μg depending on age.[91]
Reviews of clinical trials that called for long-term consumption of folic acid in amounts exceeding the UL have raised concerns.
One theory is that consumption of large amounts of folic acid leads to detectable amounts of unmetabolized folic acid circulating in blood because the enzyme dihydrofolate reductase that converts folic acid to the biologically active forms is rate limiting. Evidence of a negative health effect of folic acid in blood is not consistent, and folic acid has no known cofactor function that would increase the likelihood of a causal role for free FA in disease development.[92] However, low vitamin B12 status in combination with high folic acid intake, in addition to the previously mentioned neuropathy risk, appeared to increase the risk of cognitive impairment in the elderly.[93] And long-term use of folic acid dietary supplements in excess of 1,000 μg/day have been linked to an increase in prostate cancer risk.[53]
Food labeling
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV).
For folate labeling purposes 100% of the Daily Value was 400 μg.
As of the 27 May 2016 update, it was kept unchanged at 400 μg. [94] A table of the old and new adult Daily Values is provided at Reference Daily Intake. The original deadline to be in compliance was 28 July 2018, but on 29 September 2017 the FDA released a proposed rule that extended the deadline to 1 January 2020 for large companies and 1 January 2021 for small companies.[95] European Union regulations require that labels declare energy, protein, fat, saturated fat, carbohydrates, sugars, and salt. Voluntary nutrients may be shown if present in significant amounts. Instead of Daily Values, amounts are shown as percent of Reference Intakes (RIs). For folate, 100% RI was set at 200 μg in 2011.[96]
Food fortification
Folic acid fortification is a process where folic acid is added to flour with the intention of promoting public health through increasing blood folate levels in the populace. In the U.S., food is fortified with folic acid, only one of the many naturally occurring forms of folate, and a substance contributing only a minor amount to the folates in natural foods.[93] After the discovery of the link between insufficient folic acid and neural tube defects, governments and health organizations worldwide made recommendations concerning folic acid supplementation for women intending to become pregnant. Because the neural tube closes in the first four weeks of gestation, often before many women even know they are pregnant, many countries in time decided to implement mandatory food fortification programs. A meta-analysis of global birth prevalence of spina bifida showed that when mandatory fortification was compared to countries with voluntary fortification or no fortification program, there was a 30% reduction in live births with spina bifida.[35], with some countries reported a greater than 50% reduction.[36]
Folic acid is added to grain products in more than 80 countries,[12][34] and these fortified products make up a significant source of the population's folate intake.[97] Fortification is controversial, with issues having been raised concerning individual liberty,[93] as well as the theorized health concerns described in the Safety section. In the U.S., there is concern that the federal government mandates fortification but does not provide monitoring of potential undesirable effects of fortification.[93] The Food Fortification Initiative lists all countries in the world that conduct fortification programs,[86] and within each country, what nutrients are added to which foods. As of December 21, 2018, 81 countries required food fortification with one or more vitamins.[34] The most commonly fortified vitamin – as used in 62 countries – is folate; the most commonly fortified food is wheat flour.[34]
Australia and New Zealand
Australia and New Zealand jointly agreed to wheat flour fortification through the Food Standards Australia New Zealand in 2007. The requirement was set at 135 µg of folate per 100 g of bread. Australia implemented the program in 2009.[98] New Zealand was also planning to fortify bread (excluding organic and unleavened varieties) starting in 2009, but then opted to wait until more research was done. The Association of Bakers and the Green Party had opposed mandatory fortification, describing it as "mass medication."[99][100] Food Safety Minister Kate Wilkinson reviewed the decision to fortify in July 2009, citing as reasons to oppose claims for links between over consumption of folate with increased risk of cancer.[101] In 2012 the delayed mandatory fortification program was revoked and replaced by a voluntary program, with the hope of achieving a 50% bread fortification target.[102]
Canada
According to a Canadian survey, 58% of women said they took a folic acid containing multivitamin or a folic acid supplement as early as three months before becoming pregnant.
Women in higher income households and with more years of school education were more likely to use folic acid supplements before pregnancy, as were women with planned pregnancies and those over the age of 25.
Canadian public health efforts focused on promoting awareness of the importance of folic acid supplementation for all women of childbearing age and decreasing socio-economic inequalities by providing practical folic acid support to vulnerable groups of women.[103] Folic acid food fortification became mandatory in 1998, with the fortification of 150 µg of folic acid per 100 grams of enriched flour and uncooked cereal grains.[104] The results of folic acid fortification on the rate of neural tube defects in Canada have been positive, showing a 46% reduction in prevalence of NTDs; the magnitude of reduction was proportional to the prefortification rate of NTDs, essentially removing geographical variations in rates of NTDs seen in Canada before fortification.[105]
United Kingdom
While the Food Standards Agency recommended folic acid fortification,[106][107][108] and wheat flour is fortified with iron,[109] folic acid fortification of wheat flour is allowed voluntarily rather than required. A 2018 review by authors based in the United Kingdom strongly recommended that mandatory fortification be reconsidered as a means of reducing the risk of neural tube defects.[12]
United States

In the United States and many other countries, wheat flour is fortified with folic acid, some countries also fortify maize flour and rice.[34]
In 1996, the United States Food and Drug Administration (FDA) published regulations requiring the addition of folic acid to enriched breads, cereals, flours, corn meals, pastas, rice, and other grain products.[110][111] This ruling took effect on 1 January 1998, and was specifically targeted to reduce the risk of neural tube birth defects in newborns.[112] There were concerns expressed that the amount of folate added was insufficient.[113]
The fortification program was expected to raise a person's folic acid intake level by 70–130 µg/day;[114] however, an increase of almost double that amount was actually observed.[115] This could be from the fact that many foods are over-fortified by 160–175% over the required amount.[115] Much of the elder population take supplements that add 400 µg to their daily folic acid intake. This is a concern because 70–80% of the population have detectable levels of unmetabolized folic acid in their blood, a consequence of folic acid supplementation and fortification.[49]
The U.S. National Center for Health Statistics conducts biannual National Health and Nutrition Examination Survey (NHANES) to assess the health and nutritional status of adults and children in the United States.
Some results are reported as What We Eat In America.
The 2013–2014 survey reported that for adults ages 20 years and older, men consumed on average of 249 μg/day folate from food plus 207 μg/day of folic acid from consumption of fortified foods, for a combined total of 601 μg/day of dietary folate equivalents (DFEs; because each microgram of folic acid counts as 1.7 μg of food folate).
For women, the values are 199, 153 and 459 μg/day, respectively.
This means that fortification led to a bigger increase in folic acid intake than first projected, and that more than half the adults are consuming more than the RDA of 400 μg (as DFEs).
Even so, fewer than half of pregnant women are exceeding the pregnancy RDA of 600 μg/d.[116]
Before folic acid fortification, about 4,100 pregnancies were affected by a neural tube defect each year in the United States.
The Centers for Disease Control and Prevention reported in 2015 that since the addition of folic acid in grain-based foods as mandated by the FDA, the rate of neural tube defects dropped by 35%. This translates to an annual saving in total direct costs of approximately $508 million for the NTD-affected births that were prevented.[117][118]
History
In the 1920s, scientists believed folate deficiency and anemia were the same condition.[119] In 1931, researcher Lucy Wills made a key observation that led to the identification of folate as the nutrient required to prevent anemia during pregnancy. Wills demonstrated that anemia could be reversed with brewer's yeast.[15][120] In the late 1930s, folate was identified as the corrective substance in brewer's yeast. It was first isolated via extraction from spinach leaves by Herschel K. Mitchell, Esmond E. Snell, and Roger J. Williams in 1941.[121] Bob Stokstad isolated the pure crystalline form in 1943, and was able to determine its chemical structure while working at the Lederle Laboratories of the American Cyanamid Company.[84] This historical research project, of obtaining folic acid in a pure crystalline form in 1945, was done by the team called the "folic acid boys," under the supervision and guidance of Director of Research Dr. Yellapragada Subbarow, at the Lederle Lab, Pearl River, NY.[122]
This research subsequently led to the synthesis of the antifolate aminopterin, the first-ever anticancer drug, the clinical efficacy was proven by Sidney Farber in 1948. In the 1950s and 1960s, scientists began to discover the biochemical mechanisms of action for folate.[119] In 1960, experts first linked folate deficiency to neural tube defects.[119] In the late 1990s, U.S. scientists realized, despite the availability of folate in foods and in supplements, there was still a challenge for people to meet their daily folate requirements, which is when the US implemented the first national folate fortification program.[110][111][112] As of December 2018, 62 countries mandate food fortification with folate.[34]
Other animals
Veterinarians may test cats and dogs if a risk of folate deficiency is indicated.
Cats with exocrine pancreatic insufficiency, moreso than dogs, may have low serum folate.
In dog breeds at risk for cleft lip and cleft palate dietary folic acid supplementation significantly decreased incidence.[123]
See also
Levomefolic acid