Methylphenidate
Methylphenidate

 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmɛθəlˈfɛnɪdeɪt, -ˈfiː-/ |
| Trade names | Ritalin, Concerta, others |
| AHFS/Drugs.com | Monograph [200] |
| MedlinePlus | a682188 [201] |
| License data |
|
| Pregnancy category |
|
| Dependence liability | High[11] |
| [[LINK|lang_en|Addiction|Addiction liability]] | High[10] |
| Routes of administration | By mouth, transdermal[11] |
| Drug class | CNSstimulant |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~30% (range: 11–52%) |
| Protein binding | 10–33% |
| Metabolism | Liver (80%) mostly CES1A1-mediated |
| Elimination half-life | 2–3 hours[12] |
| Excretion | Urine (90%) |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChemCID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard(EPA) |
|
| ECHA InfoCard | 100.003.662 [214] |
| Chemical and physical data | |
| Formula | C14H19NO2 |
| Molar mass | 233.31 g/mol g·mol−1 |
| 3D model (JSmol) |
|
| Melting point | 74 °C (165 °F) [13] |
| Boiling point | 136 °C (277 °F) [13] |
SMILES
| |
InChI
| |
| (verify) [216] | |
Methylphenidate, sold under the trade name Ritalin among others, is a stimulant medication used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy.[11] It is a first line medication for ADHD.[11] It may be taken by mouth or applied to the skin.[11] Different formulations have varying durations of effect.[11]
Common side effects include trouble sleeping, anxiety and weight loss.[11] More serious side effects may include psychosis, allergic reactions, prolonged erections, abuse and heart problems. [11] Methylphenidate is believed to work by improving the action of catecholamines in the brain.[14] It achieves this by blocking dopamine and norepinephrine reuptake by neurons.[14][15] Methylphenidate is a central nervous system (CNS) stimulant of the phenethylamine and piperidine classes.[11][16]
Methylphenidate was first made in 1944 and was approved for medical use in the United States in 1955.[11][17] It was originally sold by CIBA, now Novartis Corporation.[17] It is estimated that in 2013, 2.4 billion doses of methylphenidate were taken worldwide.[18] About 80% of this was taken by people in the United States making it the 47th most prescribed medication there.[18][19] It is available as a generic medication.[11] In the United States, the wholesale cost of the immediate release formulation is less than US$0.30 per dose as of 2018.[20]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌmɛθəlˈfɛnɪdeɪt, -ˈfiː-/ |
| Trade names | Ritalin, Concerta, others |
| AHFS/Drugs.com | Monograph [200] |
| MedlinePlus | a682188 [201] |
| License data |
|
| Pregnancy category |
|
| Dependence liability | High[11] |
| [[LINK|lang_en|Addiction|Addiction liability]] | High[10] |
| Routes of administration | By mouth, transdermal[11] |
| Drug class | CNSstimulant |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~30% (range: 11–52%) |
| Protein binding | 10–33% |
| Metabolism | Liver (80%) mostly CES1A1-mediated |
| Elimination half-life | 2–3 hours[12] |
| Excretion | Urine (90%) |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChemCID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard(EPA) |
|
| ECHA InfoCard | 100.003.662 [214] |
| Chemical and physical data | |
| Formula | C14H19NO2 |
| Molar mass | 233.31 g/mol g·mol−1 |
| 3D model (JSmol) |
|
| Melting point | 74 °C (165 °F) [13] |
| Boiling point | 136 °C (277 °F) [13] |
SMILES
| |
InChI
| |
| (verify) [216] | |
Uses
Methylphenidate is most commonly used to treat ADHD and narcolepsy.[21]
Attention deficit hyperactivity disorder
The short-term benefits and cost effectiveness of methylphenidate are well established.[27][28] A number of reviews have established the safety and effectiveness of the stimulants for individuals with ADHD over several years.[29][30][30][31] A 2018 review found that it may cause both serious and non-serious adverse effects in children and adolescents.[32] The precise magnitude of improvements in ADHD symptoms and quality of life that are produced by methylphenidate treatment remains uncertain as of November 2015.[33]
Approximately 70% of those who use these stimulants see improvements in ADHD symptoms.[34][35] Children with ADHD who use stimulant medications generally have better relationships with peers and family members,[29][34] generally perform better in school, are less distractible and impulsive, and have longer attention spans.[29][34] People with ADHD have an increased risk of substance use disorders, and stimulant medications reduce this risk.[36][37] Some studies suggest that since ADHD diagnosis is increasing significantly around the world, using the drug may cause more harm than good in some populations using methylphenidate as a "study drug".[38] This applies to people who potentially may be experiencing a different issue and are misdiagnosed with ADHD.[38] People in this category can then experience negative side-effects of the drug which worsen their condition, and make it harder for them to receive adequate care as providers around them may believe the drugs are sufficient and the problem lies with the user.[38] Methylphenidate is not approved for children under six years of age.[39][40] Immediate release methylphenidate is used daily along with the longer-acting form to achieve full-day control of symptoms.[26][41] []
Narcolepsy
Narcolepsy, a chronic sleep disorder characterized by overwhelming daytime drowsiness and uncontrollable sleep, is treated primarily with stimulants. Methylphenidate is considered effective in increasing wakefulness, vigilance, and performance.[42] Methylphenidate improves measures of somnolence on standardized tests, such as the Multiple Sleep Latency Test (MSLT), but performance does not improve to levels comparable to healthy controls.[43]
Other medical uses
Methylphenidate may also be prescribed for off-label use in treatment-resistant cases of bipolar disorder and major depressive disorder.[44] It can also improve depression in several groups including stroke, cancer, and HIV-positive patients.[45] However, the use of stimulants such as methylphenidate in cases of treatment-resistant depression is controversial.[46] Stimulants may have fewer side-effects than tricyclic antidepressants in the elderly and medically ill.[47] In individuals with terminal cancer, methylphenidate can be used to counteract opioid-induced somnolence, to increase the analgesic effects of opioids, to treat depression, and to improve cognitive function.[48]
Enhancing performance
A 2015 review found that therapeutic doses of amphetamine and methylphenidate result in modest improvements in cognition, including working memory, episodic memory, and inhibitory control, in normal healthy adults;[49][50] the cognition-enhancing effects of these drugs are known to occur through the indirect activation of both dopamine receptor D1 and adrenoceptor α2 in the prefrontal cortex.[49] Methylphenidate and other ADHD stimulants also improve task saliency and increase arousal.[51][52] Stimulants such as amphetamine and methylphenidate can improve performance on difficult and boring tasks[53][51][52] and are used by some students as a study and test-taking aid.[54][38] Based upon studies of self-reported illicit stimulant use, performance-enhancing use, rather than use as a recreational drug, is the primary reason that students use stimulants.[55]
Excessive doses of methylphenidate, above the therapeutic range, can interfere with working memory and cognitive control.[51][52] Like amphetamine and bupropion, methylphenidate increases stamina and endurance in humans primarily through reuptake inhibition of dopamine in the central nervous system.[56] Similar to the loss of cognitive enhancement when using large amounts, large doses of methylphenidate can induce side effects that impair athletic performance, such as rhabdomyolysis and hyperthermia.[57] While literature suggests it might improve cognition, most authors agree that using the drug recreationally as a study aid when ADHD diagnosis is not present does not actually improve GPA.[38] Moreover, it has been suggested that students who use the drug for studying may be self-medicating for potentially deeper underlying issues.[38]
Contraindications
The US FDA gives methylphenidate a pregnancy category of C, and women are advised to only use the drug if the benefits outweigh the potential risks.[59] Not enough animal and human studies have been conducted to conclusively demonstrate an effect of methylphenidate on fetal development. In 2007, empirical literature included 63 cases of prenatal exposure to methylphenidate across three empirical studies.[60]
Adverse effects

Addiction experts in psychiatry, chemistry, pharmacology, forensic science, epidemiology, and the police and legal services engaged in delphic analysis regarding 20 popular recreational drugs. Methylphenidate was ranked 13th in dependence, 12th in physical harm, and 18th in social harm.[61]
Methylphenidate is generally well tolerated.[62][63] The most commonly observed adverse effects with a frequency greater than placebo include appetite loss, dry mouth, anxiety/nervousness, nausea, and insomnia. Gastrointestinal adverse effects may include abdominal pain and weight loss. Nervous system adverse effects may include akathisia (agitation/restlessness), irritability, dyskinesia (tics), lethargy (drowsiness/fatigue), and dizziness. Cardiac adverse effects may include palpitations, changes in blood pressure and heart rate (typically mild), tachycardia (rapid resting heart rate), and Raynaud's phenomenon (reduced blood flow to the hands and feet).[64] Ophthalmologic adverse effects may include blurred vision and dry eyes, with less frequent reports of diplopia and mydriasis.[65] Other adverse effects may include depression, emotional lability, confusion, and bruxism. Hyperhidrosis (increased sweating) is common. Chest pain is rarely observed.[66]
Hypersensitivity (including skin rash, urticaria, and fever) is sometimes reported. The Daytrana patch has a much higher rate of dermal reactions than oral methylphenidate.[70]
Methylphenidate can worsen psychosis in people who are psychotic, and in very rare cases it has been associated with the emergence of new psychotic symptoms.[71] It should be used with extreme caution in people with bipolar disorder due to the potential induction of mania or hypomania.[72] There have been very rare reports of suicidal ideation, but evidence does not support a link.[67] Logorrhea is occasionally reported. Libido disorders, disorientation, and hallucinations are very rarely reported. Priapism is a very rare adverse event that can be potentially serious.[73]
USFDA-commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of methylphenidate or other ADHD stimulants.[74]
Because some adverse effects may only emerge during chronic use of methylphenidate, a constant watch for adverse effects is recommended.[75]
Overdose
The symptoms of a moderate acute overdose on methylphenidate primarily arise from central nervous system overstimulation; these symptoms include: vomiting, agitation, tremors, hyperreflexia, muscle twitching, euphoria, confusion, hallucinations, delirium, hyperthermia, sweating, flushing, headache, tachycardia, heart palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes.[57][76] A severe overdose may involve symptoms such as hyperpyrexia, sympathomimetic toxidrome, convulsions, paranoia, stereotypy (a repetitive movement disorder), rapid muscle breakdown, coma, and circulatory collapse.[57][76][77] A methylphenidate overdose is rarely fatal with appropriate care.[77] Severe toxic reactions involving abscess and necrosis have been reported following injection of methylphenidate tablets into an artery.[78]
Treatment of a methylphenidate overdose typically involves the application of benzodiazepines, with antipsychotics, α-adrenoceptor agonists, and propofol serving as second-line therapies.[77]
Addiction and dependence
ΔFosB accumulation from excessive drug use Top: this depicts the initial effects of high dose exposure to an addictive drug on gene expression in the nucleus accumbens for various Fos family proteins (i.e., c-Fos, FosB, ΔFosB, Fra1, and Fra2). Bottom: this illustrates the progressive increase in ΔFosB expression in the nucleus accumbens following repeated twice daily drug binges, where these phosphorylated (35–37 kilodalton) ΔFosB isoforms persist in the D1-type medium spiny neurons of the nucleus accumbens for up to 2 months.[79][80] |
Methylphenidate is a stimulant with an addiction liability and dependence liability similar to amphetamine. It has moderate liability among addictive drugs;[81][82] accordingly, addiction and psychological dependence are possible and likely when methylphenidate is used at high doses as a recreational drug.[82][83] When used above the medical dose range, stimulants are associated with the development of stimulant psychosis.[84] As with all addictive drugs, the overexpression of ΔFosB in D1-type medium spiny neurons in the nucleus accumbens is implicated in methylphenidate addiction.[83][85]
Methylphenidate has shown some benefits as a replacement therapy for individuals who are addicted to and dependent upon methamphetamine.[86] Methylphenidate and amphetamine have been investigated as a chemical replacement for the treatment of cocaine addiction[87][88][89][90] in the same way that methadone is used as a replacement drug for physical dependence upon heroin. Its effectiveness in treatment of cocaine or psychostimulant addiction or psychological dependence has not been proven and further research is needed.[91]
Biomolecular mechanisms
Methylphenidate has the potential to induce euphoria due to its pharmacodynamic effect (i.e., dopamine reuptake inhibition) in the brain's reward system.[85] At therapeutic doses, ADHD stimulants do not sufficiently activate the reward system, or the reward pathway in particular, to the extent necessary to cause persistent increases in ΔFosB gene expression in the D1-type medium spiny neurons of the nucleus accumbens;[82][85][92] consequently, when taken as directed in doses that are commonly prescribed for the treatment of ADHD, methylphenidate use lacks the capacity to cause an addiction.[82][85][92] However, when methylphenidate is used at sufficiently high recreational doses through a bioavailable route of administration (e.g., insufflation or intravenous administration), particularly for use of the drug as a euphoriant, ΔFosB accumulates in the nucleus accumbens.[82][85] Hence, like any other addictive drug, regular recreational use of methylphenidate at high doses eventually gives rise to ΔFosB overexpression in D1-type neurons which subsequently triggers a series of gene transcription-mediated signaling cascades that induce an addiction.[85][92][93]
Interactions
Methylphenidate may inhibit the metabolism of coumarin anticoagulants, certain anticonvulsants, and some antidepressants (tricyclic antidepressants and selective serotonin reuptake inhibitors). Concomitant administration may require dose adjustments, possibly assisted by monitoring of plasma drug concentrations.[63] There are several case reports of methylphenidate inducing serotonin syndrome with concomitant administration of antidepressants.[94][95][96][97]
When methylphenidate is coingested with ethanol, a metabolite called ethylphenidate is formed via hepatic transesterification,[98][99] not unlike the hepatic formation of cocaethylene from cocaine and ethanol. The reduced potency of ethylphenidate and its minor formation means it does not contribute to the pharmacological profile at therapeutic doses and even in overdose cases ethylphenidate concentrations remain negligible.[100][99]
Coingestion of alcohol (ethanol) also increases the blood plasma levels of d-methylphenidate by up to 40%.[101]
Liver toxicity from methylphenidate is extremely rare, but limited evidence suggests that intake of β-adrenergic agonists with methylphenidate may increase the risk of liver toxicity.[102]
Pharmacology
Pharmacodynamics
| Neurotransmitter transporter | Measure (units) | dl-MPH | d-MPH | l-MPH |
|---|---|---|---|---|
| DAT | Ki (nM) | 121 | 161 | 2250 |
| IC50 (nM) | 20 | 23 | 1600 | |
| NET | Ki(nM) | 788 | 206 | 10000 |
| IC50(nM) | 51 | 39 | 980 | |
| SERT | Ki(nM) | 10000 | 10000 | 6700 |
| IC50(nM) | — | 10000 | 10000 | |
| GPCR | Measure (units) | dl-MPH | d-MPH | l-MPH |
| 5-HT1A | Ki (nM) | 5000 | 3400 | 10000 |
| IC50 (nM) | 10000 | 6800 | 10000 | |
| 5-HT2B | Ki(nM) | 10000 | 4700 | 10000 |
| IC50(nM) | 10000 | 4900 | 10000 |
Methylphenidate primarily acts as a norepinephrine–dopamine reuptake inhibitor (NDRI). It is a benzylpiperidine and phenethylamine derivative which also shares part of its basic structure with catecholamines.
Methylphenidate is a psychostimulant and increases the activity of the central nervous system through inhibition on reuptake of the neurotransmitters norepinephrine and dopamine. As models of ADHD suggest, it is associated with functional impairments in some of the brain's neurotransmitter systems, particularly those involving dopamine in the mesocortical and mesolimbic pathways and norepinephrine in the prefrontal cortex and locus coeruleus.[106] Psychostimulants like methylphenidate and amphetamine may be effective in treating ADHD because they increase neurotransmitter activity in these systems. When reuptake of those neurotransmitters is halted, its concentration and effects in the synapse increase and last longer, respectively. Therefore, methylphenidate is called a norepinephrine–dopamine reuptake inhibitor.[100] By increasing the effects of norepinephrine and dopamine, methylphenidate increased the activity of the central nervous system and produced effects such as increased alertness, combated fatigue and improved attention.[107] [106]
Methylphenidate is most active at modulating levels of dopamine (DA) and to a lesser extent norepinephrine.[108] Methylphenidate binds to and blocks dopamine transporters (DAT) and norepinephrine transporters.[109] Variability exists between DAT blockade, and extracellular dopamine, leading to the hypothesis that methylphenidate amplifies basal dopamine activity, leading to nonresponse in those with low basal DA activity.[110] On average, methylphenidate elicits a 3–4 times increase in dopamine and norepinephrine in the striatum and prefrontal cortex.[111] Magnetic resonance imaging (MRI) studies suggest that long-term treatment with ADHD stimulants (specifically, amphetamine and methylphenidate) decreases abnormalities in brain structure and function found in subjects with ADHD.[112][113][114]
Both amphetamine and methylphenidate are predominantly dopaminergic drugs, yet their mechanisms of action are distinct. Methylphenidate acts as a norepinephrine–dopamine reuptake inhibitor while amphetamine is both a releasing agent and reuptake inhibitor of dopamine and norepinephrine. Methylphenidate's mechanism of action in the release of dopamine and norepinephrine is fundamentally different from most other phenethylamine derivatives, as methylphenidate is thought to increase neuronal firing rate,[115][116][117][118] whereas amphetamine reduces firing rate, but causes monoamine release by reversing the flow of the monoamines through monoamine transporters via a diverse set of mechanisms, including TAAR1 activation and modulation of VMAT2 function, among other mechanisms.[119][120][121] The difference in mechanism of action between methylphenidate and amphetamine results in methylphenidate inhibiting amphetamine's effects on monoamine transporters when they are co-administered.[119]
Methylphenidate has both dopamine transporter and norepinephrine transporter binding affinity, with the dextromethylphenidate enantiomers displaying a prominent affinity for the norepinephrine transporter. Both the dextrorotary and levorotary enantiomers displayed receptor affinity for the serotonergic 5HT1A and 5HT2B subtypes, though direct binding to the serotonin transporter was not observed.[105] A later study confirmed the d-threo- enantiomer binding to the 5HT1A receptor, but no significant activity on the 5HT2B receptor was found.[122]
Methylphenidate may protect neurons from the neurotoxic effects of Parkinson's disease and methamphetamine abuse.[123] The hypothesized mechanism of neuroprotection is through inhibition of methamphetamine/DAT interactions, and through reducing cytosolic dopamine, leading to decreased production of dopamine related reactive oxygen species.[124]
Pharmacokinetics
Methylphenidate taken orally has a bioavailability of 11–52% with a duration of peak action around 2–4 hours for instant release (i.e. Ritalin), 3–8 hours for sustained release (i.e. Ritalin SR), and 8–12 hours for extended release (i.e. Concerta). The half-life of methylphenidate is 2–3 hours, depending on the individual. The peak plasma time is achieved at about 2 hours.[12] Methylphenidate has a low plasma protein binding of 10-33% and a volume of distribution of 2.65L/kg.[126]
Dextromethylphenidate is much more bioavailable than levomethylphenidate when administered orally, and is primarily responsible for the psychoactivity of racemic methylphenidate.[12]
Contrary to the expectation, taking methylphenidate with a meal speeds absorption.[127]
Methylphenidate is metabolized into ritalinic acid by CES1A1, enzymes in the liver. Dextromethylphenidate is selectively metabolized at a slower rate than levomethylphenidate.[128] 97% of the metabolised drug is excreted in the urine, and between 1 and 3% is excreted in the faeces. A small amount, less than 1%, of the drug is excreted in the urine in its unchanged form.[126]
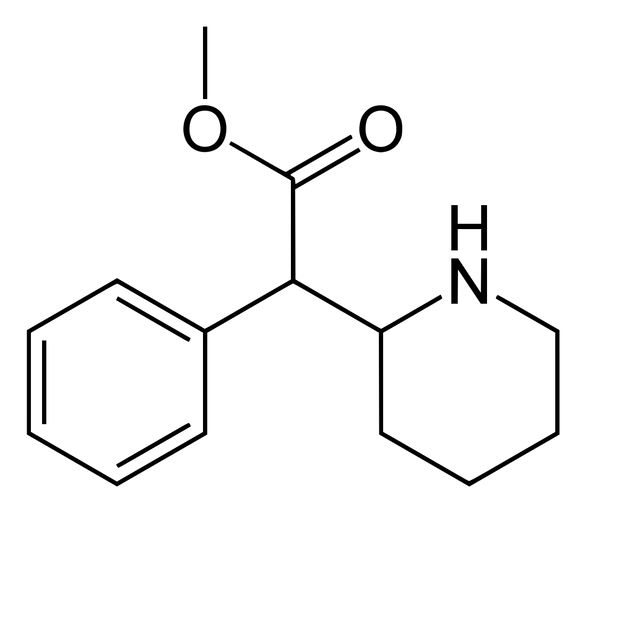
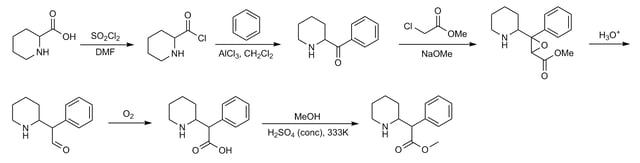
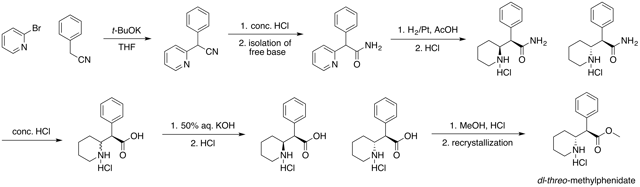
Chemistry
Method 3: Another synthesis route of methylphenidate which applies Darzens reaction to obtain aldehyde as an intermediate. This route is significant for its selectivity.
Method 2: Classic methylphenidate synthesis[132]
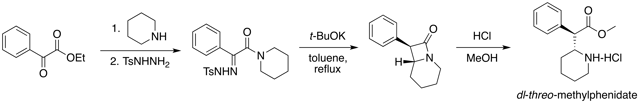
Method 1: Methylphenidate preparation elucidated by Axten et al. (1998)[131] via Bamford-Stevens reaction.
Four isomers of methylphenidate are possible, since the molecule has two chiral centers. One pair of threo isomers and one pair of erythro are distinguished, from which primarily d-threo-methylphenidate exhibits the pharmacologically desired effects.[108][129] The erythro diastereomers are pressor amines, a property not shared with the threo diastereomers. When the drug was first introduced it was sold as a 4:1 mixture of erythro:threo diastereomers, but it was later reformulated to contain only the threo diastereomers. "TMP" refers to a threo product that does not contain any erythro diastereomers, i.e. (±)-threo-methylphenidate. Since the threo isomers are energetically favored, it is easy to epimerize out any of the undesired erythro isomers. The drug that contains only dextrorotatory methylphenidate is sometimes called d-TMP, although this name is only rarely used and it is much more commonly referred to as dexmethylphenidate, d-MPH, or d-threo-methylphenidate. A review on the synthesis of enantiomerically pure (2R,2'R)-(+)-threo-methylphenidate hydrochloride has been published.[130]
Detection in biological fluids
The concentration of methylphenidate or ritalinic acid, its major metabolite, may be quantified in plasma, serum or whole blood in order to monitor compliance in those receiving the drug therapeutically, to confirm the diagnosis in potential poisoning victims or to assist in the forensic investigation in a case of fatal overdosage.[133]
History
Methylphenidate was synthesized by Ciba (now Novartis) chemist Leandro Panizzon. He named the drug after his wife, nicknamed Rita, who used Ritalin to compensate for low blood pressure.[136]
Originally it was marketed as a mixture of two racemates, 80% (±)-erythro and 20% (±)-threo. Subsequent studies of the racemates showed that the central stimulant activity is associated with the threo racemate and were focused on the separation and interconversion of the erythro isomer into the more active threo isomer.[137][138][139]
Methylphenidate was first used to allay barbiturate-induced coma, narcolepsy and depression.[140] It was later used to treat memory deficits in the elderly.[141] Beginning in the 1960s, it was used to treat children with ADHD based on earlier work starting with the studies by American psychiatrist Charles Bradley[142] on the use of psychostimulant drugs, such as Benzedrine, with then called "maladjusted children".[143] Production and prescription of methylphenidate rose significantly in the 1990s, especially in the United States, as the ADHD diagnosis came to be better understood and more generally accepted within the medical and mental health communities.[144]
In 2000 ALZA Corporation received US Food and Drug Administration (FDA) approval to market "Concerta", an extended-release form of methylphenidate.[145]
Society and culture
Names
German "Ritalin" brand methylphenidate
Methylphenidate is produced in the United States, Mexico, Spain, Sweden, Pakistan, and India. It is also sold in Canada, Australia, the United Kingdom, Spain, Germany, Belgium, Brazil, Portugal, Argentina, Thailand, and several other European countries (although in much lower volumes than in the United States). Brand names for methylphenidate include Ritalin, Concerta, Inspiral, Addwize, Aptensio, Biphentin, Daytrana, Equasym, Medikinet, Metadate, Methylin, and Quillivant. Generic forms are produced by numerous pharmaceutical companies throughout the world.
Available forms

Clockwise from top: Concerta 18 mg, Medikinet 30 mg, Methylphenidat TAD 10 mg, Ritalin 10 mg, Medikinet XL 30 mg.
Methylphenidate is available in numerous forms, and a doctor will determine the appropriate formulation of the drug to prescribe based on the patient's history, the doctor's experiences treating other patients with methylphenidate products, and product pricing/availability. Currently available forms include a variety of tablets and capsules, an adhesive-based matrix transdermal system (transdermal patch), and an oral suspension (liquid syrup).
The dextrorotary enantiomer of methylphenidate, known as dexmethylphenidate, is sold as a generic and under the brand names Focalin and Attenade in both an immediate-release and an extended-release form. In some circumstances it may be prescribed instead of methylphenidate, however it has no significant advantages over methylphenidate at equipotent dosages and so it is sometimes considered to be an example of an evergreened drug.[146]
Immediate-release
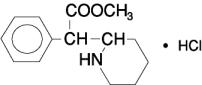
Structural formula for the substance among Ritalin tablet series. (Ritalin, Ritalin LA, Ritalin SR.) The volume of distribution was 2.65±1.11 L/kg for d-methylphenidate and 1.80±0.91 L/kg for l-methylphenidate subsequent to swallow of Ritalin tablet.[147]
Methylphenidate was originally available as an immediate-release racemic mixture formulation under the Novartis trademark name Ritalin, although a variety of generics are now available, some under other brand names. Generic brand names include Ritalina, Rilatine, Attenta, Medikinet, Metadate, Methylin, Penid, Tranquilyn, and Rubifen.
Extended-release
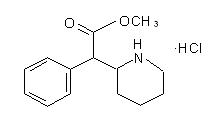
Structural formula for the substance inside Concerta tablet. Following administration of CONCERTA®, plasma concentrations of the l-isomer were approximately 1/40 the plasma concentrations of the d-isomer.[148]
Extended-release methylphenidate products include:(see chart, below)
| Brand name(s) | Generic name(s)[1] | Duration | Dosage form |
|---|---|---|---|
| Aptensio XR (US); Biphentin (CA) | Currently unavailable | 12 hours[2] | XR capsule |
| Concerta (US/CA); Concerta XL (UK) | methylphenidate ER (US/CA);[3] methylphenidate ER‑C (CA)[4] | 12 hours[155] | OROS tablet |
| Quillivant XR (US) | Currently unavailable | 12 hours[155] | oral suspension |
| Daytrana (US) | Currently unavailable | 11 hours[156] | transdermal patch |
| Metadate CD (US); Equasym XL (UK) | methylphenidate ER (US)[5] | 8–10 hours[155] | CD/XL capsule |
| QuilliChew ER (US) | Currently unavailable | 8 hours[157] | chewable tablet |
| Ritalin LA (US); Medikinet XL (UK) | methylphenidate ER (US)[6] | 8 hours[155] | ER capsule |
| Ritalin SR (US/CA/UK); Rubifen SR (NZ) | Metadate ER (US);[7] Methylin ER (US);[8] methylphenidate SR (US/CA)[9] | 5–8 hours[155] | CR tablet |
Concerta tablets are marked with the letters "ALZA" and followed by: "18", "27", "36", or "54", relating to the mg dosage strength. Approximately 22% of the dose is immediate release,[158] and the remaining 78% of the dose is released over 10–12 hours post ingestion, with an initial increase over the first 6 to 7 hours, and subsequent decline in released drug.[159]
Ritalin LA capsules are marked with the letters "NVR" (abbrev.: Novartis) and followed by: "R20", "R30", or "R40", depending on the (mg) dosage strength. Ritalin LA[64] provides two standard doses – half the total dose being released immediately and the other half released four hours later. In total, each capsule is effective for about eight hours.
Metadate CD capsules contain two types of beads; 30% are immediate release, and the other 70% are evenly sustained release.[160]
Quillivant XR is an extended-release oral suspension (after reconstitution with water): 25 mg per 5 mL (5 mg per mL). It was designed and is patented and made by Pfizer. The medication comes in various sizes from 60ml to 180ml (after reconstitution). Each bottle is shipped with the medication in powder form containing roughly 20% instant-release and 80% extended-release methylphenidate, to which water must be added by the pharmacist in an amount corresponding with the total intended volume of the bottle. The bottle must be shaken vigorously for ten seconds prior to administration via included oral syringe to ensure proper ratio.[161]
Cost

Ritalin 10 mg tablet
Generic immediate-release methylphenidate is relatively inexpensive. The average wholesale cost is about US$0.15 per defined daily dose (retail pharmacies normally charge more).[162] However, the most expensive brand-name extended-release tablets may retail for as much as $12.40 per defined daily dose.[163]
There are two main reasons for this price difference:
Generic formulations are less expensive than brand-name formulations.
Immediate-release tablets are less expensive than 8-hour extended-release tablets, which are much less expensive than 12-hour extended-release tablets.
Legal status

Legal warning printed on Ritalin packaging
Internationally, methylphenidate is a Schedule II drug under the Convention on Psychotropic Substances.[164]
In the United States, methylphenidate is classified as a Schedule II controlled substance, the designation used for substances that have a recognized medical value but present a high potential for abuse.
In the United Kingdom, methylphenidate is a controlled 'Class B' substance. Possession without prescription carries a sentence up to 5 years or an unlimited fine, or both; supplying methylphenidate is 14 years or an unlimited fine, or both.[165]
In Canada, methylphenidate is listed in Schedule III of the Controlled Drugs and Substances Act and is illegal to possess without a prescription, with unlawful possession punishable by up to three years imprisonment, or (via summary conviction) by up to one year imprisonment and/or fines of up to two thousand dollars. Unlawful possession for the purpose of trafficking is punishable by up to ten years imprisonment, or (via summary conviction) by up to eighteen months imprisonment.[166]
In New Zealand, methylphenidate is a 'class B2 controlled substance'. Unlawful possession is punishable by six-month prison sentence and distribution by a 14-year sentence.
In Australia, methylphenidate is a 'Schedule 8' controlled substance.[167] Such drugs must be kept in a lockable safe until dispensed and possession without prescription is punishable by fines and imprisonment.
In Sweden, methylphenidate is a List II controlled substance with recognized medical value. Possession without a prescription is punishable by up to three years in prison.[168]
In France, methylphenidate is covered by the "narcotics" schedule, prescription and distribution conditions are restricted with hospital-only prescription for the initial treatment and yearly consultations.[169]
In India, methylphenidate is a schedule X drug and is controlled by the Drugs and Cosmetics Rule, 1945. It is dispensed only by physician's prescription.[170] Legally, 2 grams of methylphenidate are classified as a small quantity, and 50 grams as a large or commercial quantity.[171]
In Hong Kong, methylphenidate is controlled under the schedule 1 of the Dangerous Drugs Ordinance (Cap. 134).[172]
Controversy
Methylphenidate has been the subject of controversy in relation to its use in the treatment of ADHD. The prescription of psychostimulant medication to children to reduce ADHD symptoms has been a major point of criticism.[173] The contention that methylphenidate acts as a gateway drug has been discredited by multiple sources,[174] according to which abuse is statistically very low and "stimulant therapy in childhood does not increase the risk for subsequent drug and alcohol abuse disorders later in life".[175] A study found that ADHD medication was not associated with increased risk of cigarette use, and in fact stimulant treatments such as Ritalin seemed to lower this risk.[176]
One of the highest use of methylphenidate medication is in Iceland,[177] where research shows that the drug was the most commonly abused substance among intravenous substance abusers.[178] The study involved 108 intravenous substance abusers and 88% of them had injected methylphenidate within the last 30 days and for 63% of them, methylphenidate was the most preferred substance.
Treatment of ADHD by way of methylphenidate has led to legal actions, including malpractice suits regarding informed consent, inadequate information on side effects, misdiagnosis, and coercive use of medications by school systems.[179]
In the US and the UK, it is approved for use in children and adolescents. In the US, the Food and Drug Administration approved the use of methylphenidate in 2008 for use in treating adult ADHD.[180] In the UK, while not licensed for use in adult ADHD, NICE guidelines suggest it be prescribed off-license for the condition.[181] Methylphenidate has been approved for adult use in the treatment of narcolepsy.[182]
Research
Methylphenidate may provide possible protection from methamphetamine induced dopamine neuron damage and possible protection from Parkinson disease.[185]