Methadone
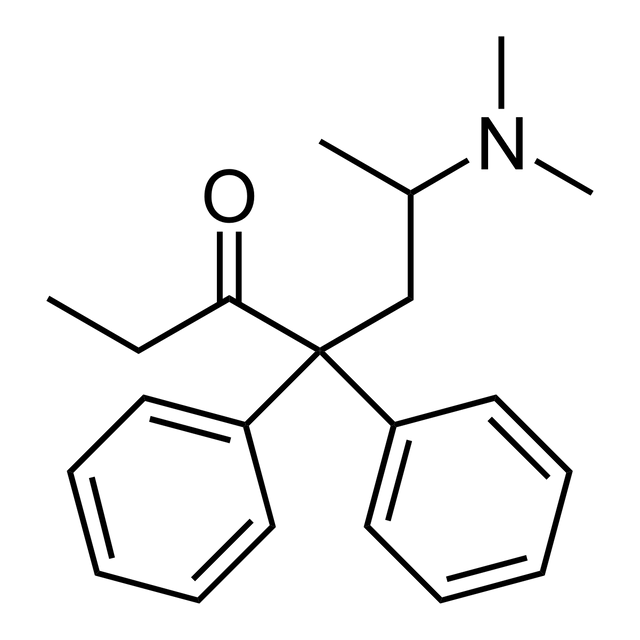
Methadone

| Clinical data | |
|---|---|
| Trade names | Dolophine, Methadose, others |
| AHFS/Drugs.com | |
| MedlinePlus | |
| Pregnancy category | |
| [[LINK|lang_en|Addiction|Addiction liability]] | High[1] |
| Routes of administration | By mouth, intravenous, insufflation, sublingual, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 15-20% subcutaneous[2]100% intravenous[2]41–99% (by mouth)[2] |
| Protein binding | 85–90%[2] |
| Metabolism | Liver(CYP3A4,CYP2B6andCYP2D6-mediated)[2][3] |
| Onset of action | Rapid[4] |
| Elimination half-life | 15 to 55 hours[3] |
| Duration of action | Single dose: 4–8 hProlonged use: • Withdrawal prevention: 1–2 days[4]• Pain relief: 8–12 hours[4][5] |
| Excretion | Urine, faeces[3] |
| Identifiers | |
| CAS Number | |
| PubChem | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard | |
| ECHA InfoCard | 100.000.907[126] |
| Chemical and physical data | |
| Formula | |
| Molar mass | 309.445 g/mol g·mol |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
Methadone, sold under the brand name Dolophine among others, is an opioid used for opioid maintenance therapy in opioid dependence and for chronic pain management.[4] Detoxification using methadone can be accomplished in less than a month, or it may be done gradually over as long as six months.[4] While a single dose has a rapid effect, maximum effect can take up to five days of use.[4] The pain-relieving effects last about six hours after a single dose.[4][6] After long-term use, in people with normal liver function, effects last 8 to 36 hours.[4][5] Methadone is usually taken by mouth and rarely by injection into a muscle or vein.[4]
Side effects are similar to those of other opioids.[4] These frequently includes dizziness, sleepiness, vomiting, and sweating.[4] Serious risks include opioid abuse and a decreased effort to breathe.[4] Abnormal heart rhythms may also occur due to a prolonged QT interval.[4] The number of deaths in the United States involving methadone poisoning declined from 4,418 in 2011[7] to 3,300 in 2015.[8] Risks are greater with higher doses.[9] Methadone is made by chemical synthesis and acts on opioid receptors.[4]
Methadone was developed in Germany around 1937 to 1939 by Gustav Ehrhart and Max Bockmühl.[10][11] It was approved for use in the United States in 1947.[4] Methadone is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[12] In 2013, about 41,400 kilograms were manufactured globally.[13] It is regulated similarly to other narcotic drugs.[14] It is not particularly expensive in the United States.[15]
| Clinical data | |
|---|---|
| Trade names | Dolophine, Methadose, others |
| AHFS/Drugs.com | |
| MedlinePlus | |
| Pregnancy category | |
| [[LINK|lang_en|Addiction|Addiction liability]] | High[1] |
| Routes of administration | By mouth, intravenous, insufflation, sublingual, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokineticdata | |
| Bioavailability | 15-20% subcutaneous[2]100% intravenous[2]41–99% (by mouth)[2] |
| Protein binding | 85–90%[2] |
| Metabolism | Liver(CYP3A4,CYP2B6andCYP2D6-mediated)[2][3] |
| Onset of action | Rapid[4] |
| Elimination half-life | 15 to 55 hours[3] |
| Duration of action | Single dose: 4–8 hProlonged use: • Withdrawal prevention: 1–2 days[4]• Pain relief: 8–12 hours[4][5] |
| Excretion | Urine, faeces[3] |
| Identifiers | |
| CAS Number | |
| PubChem | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard | |
| ECHA InfoCard | 100.000.907[126] |
| Chemical and physical data | |
| Formula | |
| Molar mass | 309.445 g/mol g·mol |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
Medical uses
Methadone maintenance
Methadone is used for the treatment of opioid use disorder. It may be used as a maintenance therapy or in shorter periods for detoxification to manage opioid withdrawal symptoms.
A 2009 Cochrane review found methadone was effective in retaining people in treatment and in the reduction or cessation of heroin use as measured by self-report and urine/hair analysis but did not affect criminal activity or risk of death.[16]
Treatment of opioid-dependent persons with methadone follows one of two routes: maintenance or detoxification.[17] Methadone maintenance therapy (MMT) usually takes place in outpatient settings.
It is usually prescribed as a single daily dose medication for those who wish to abstain from illicit opioid use.
Treatment models for MMT differ.
It is not uncommon for treatment recipients to be administered methadone in a specialist clinic, where they are observed for around 15–20 minutes post dosing, to reduce risk of diversion of medication.[18]
The duration of methadone treatment programs range from a few months to several years.
Given opioid dependence is characteristically a chronic relapsing/remitting disorder, MMT may be lifelong.
The length of time a person remains in treatment depends on a number of factors.
While starting doses may be adjusted based on the amount of opioids reportedly used, most clinical guidelines suggest doses start low (e.g. at doses not exceeding 40 mg daily) and are incremented gradually.[19][20]
Methadone maintenance has been shown to reduce the transmission of blood borne viruses associated with opioid injection, such as hepatitis B and C, and/or HIV.[19] The principal goals of methadone maintenance are to relieve opioid cravings, suppress the abstinence syndrome, and block the euphoric effects associated with opioids.
Chronic methadone dosing will eventually lead to neuroadaptation, characterised by a syndrome of tolerance and withdrawal (dependence).
However, when used correctly in treatment, maintenance therapy has been found to be medically safe, non-sedating, and can provide a slow recovery from opioid addiction.[19] Methadone has been widely used for pregnant women addicted to opioids.[19]
Opioid detoxification
Methadone is approved in the US, and many other parts of the world, for the treatment of opioid addiction.
Its use for the treatment of addiction is usually strictly regulated.
In the US, outpatient treatment programs must be certified by the federal Substance Abuse and Mental Health Services Administration (SAMHSA) and registered by the Drug Enforcement Administration (DEA) in order to prescribe methadone for opioid addiction.
Pain
Adverse effects

Addiction experts in psychiatry, chemistry, pharmacology, forensic science, epidemiology, and the police and legal services engaged in delphic analysis regarding 20 popular recreational drugs. Street methadone was ranked 4th in dependence, 5th in physical harm, and 5th in social harm.[25]
Adverse effects of methadone include:
Sedation
Flushing[27]
Heat intolerance
Weakness[27]
Chronic fatigue, sleepiness[27] and exhaustion
Sleep problems such as drowsiness,[26] trouble falling asleep (Insomnia),[27][28] and trouble staying asleep[27]
Constricted pupils
Low blood pressure
Headache[27]
Heart problems such as chest pain[26][28] or fast/pounding heartbeat[26][28][29]
Respiratory problems such as trouble breathing,[26][28] slow or shallow breathing (hypoventilation),[26][28] light-headedness,[26][28][29] or fainting[26][28]
Weight loss or weight gain[27]
Memory loss
Stomach pains[27]
Itching
Difficulty urinating[27]
Swelling of the hands, arms, feet, and legs[27]
Feeling restless[26] or agitated
Mood changes,[27] euphoria, disorientation
Blurred vision[27]
Decreased libido,[26][27] missed menstrual periods,[27] difficulty in reaching orgasm,[26] or impotence[26][27]
Skin rash
Seizures
Central sleep apnea
Withdrawal symptoms
Physical symptoms
Lightheadedness[31]
Mydriasis (dilated pupils)[31]
Photophobia (sensitivity to light)
Hyperventilation syndrome (breathing that is too fast/deep)
Runny nose[32]
Yawning
Sneezing[32]
Fever[32]
Sweating[31]
Chills[32]
Akathisia (restlessness)
Tachycardia (fast heartbeat)[32]
Aches[31] and pains, often in the joints or legs
Elevated pain sensitivity
Blood pressure that is too high (hypertension, may cause stroke)
Cognitive symptoms
Suicidal ideation
Susceptibility to cravings[31]
Depression[31]
Spontaneous orgasm
Prolonged insomnia
Delirium
Auditory hallucinations
Visual hallucinations
Increased perception of odors (olfaction), real or imagined
Marked decrease or increase in sex drive
Agitation
Panic disorder
Nervousness[31]
Paranoia
Delusions
Apathy
Anorexia (symptom)
Methadone withdrawal symptoms are reported as being significantly more protracted than withdrawal from opioids with shorter half-lives.
Methadone is sometimes administered as an oral liquid.
Methadone has been implicated in contributing to significant tooth decay. Methadone causes dry mouth, reducing the protective role of saliva in preventing decay. Other putative mechanisms of methadone-related tooth decay include craving for carbohydrates related to opioids, poor dental care, and general decrease in personal hygiene. These factors, combined with sedation, have been linked to the causation of extensive dental damage.[33][34]
Black box warning
Methadone has the following US FDA black box warning:[35]
Risk of addiction and abuse
Potentially fatal respiratory depression
Lethal overdose in accidental ingestion
QT prolongation
Neonatal opioid withdrawal syndrome in children of pregnant women
CYP450 drug interactions
Risks when used with benzodiazepines and other CNS suppressants, including alcohol.
A certified opioid treatment program is required under federal law (42 CFR 8.12) when dispensing methadone for the treatment of opioid addiction or detoxification.
Overdose
Most people who have overdosed on methadone may show some of the following symptoms:
The respiratory depression of an overdose can be treated with naloxone.[32] Naloxone is preferred to the newer, longer acting antagonist naltrexone. Despite methadone's much longer duration of action compared to either heroin and other shorter-acting agonists, and the need for repeat doses of the antagonist naloxone, it is still used for overdose therapy. As naltrexone has a longer half-life, it is more difficult to titrate. If too large a dose of the opioid antagonist is given to a dependent person, it will result in withdrawal symptoms (possibly severe). When using naloxone, the naloxone will be quickly eliminated and the withdrawal will be short lived. Doses of naltrexone take longer to be eliminated from the person's system. A common problem in treating methadone overdoses is that, given the short action of naloxone (versus the extremely longer-acting methadone), a dosage of naloxone given to a methadone-overdosed person will initially work to bring the person out of overdose, but once the naloxone wears off, if no further naloxone is administered, the person can go right back into overdose (based upon time and dosage of the methadone ingested).
Tolerance and dependence
As with other opioid medications, tolerance and dependence usually develop with repeated doses.
There is some clinical evidence that tolerance to analgesia is less with methadone compared to other opioids; this may be due to its activity at the NMDA receptor.
Tolerance to the different physiological effects of methadone varies; tolerance to analgesic properties may or may not develop quickly, but tolerance to euphoria usually develops rapidly, whereas tolerance to constipation, sedation, and respiratory depression develops slowly (if ever).[38]
Driving
Methadone treatment may impair driving ability.[39] Drug abusers had significantly more involvement in serious crashes than non-abusers in a study by the University of Queensland.
In the study of a group of 220 drug abusers, most of them poly-drug abusers, 17 were involved in crashes killing people, compared with a control group of other people randomly selected having no involvement in fatal crashes.[40] However, there have been multiple studies verifying the ability of methadone maintenance patients to drive.[41] In the UK, persons who are prescribed oral Methadone can continue to drive after they have satisfactorily completed an independent medical examination which will include a urine screen for drugs. The license will be issued for 12 months at a time and even then, only following a favourable assessment from their own doctor. [undefined] Individuals who are prescribed methadone for either IV or IM administration cannot drive in the UK, mainly due to the increased sedation effects that this route of use can cause.
Mortality
In the United States, deaths linked to methadone more than quadrupled in the five-year period between 1999 and 2004.
According to the U.S.
National Center for Health Statistics,[43] as well as a 2006 series in the Charleston Gazette (West Virginia),[44] medical examiners listed methadone as contributing to 3,849 deaths in 2004. That number was up from 790 in 1999. Approximately 82 percent of those deaths were listed as accidental, and most deaths involved combinations of methadone with other drugs (especially benzodiazepines).
Although deaths from methadone are on the rise, methadone-associated deaths are not being caused primarily by methadone intended for methadone treatment programs, according to a panel of experts convened by the Substance Abuse and Mental Health Services Administration, which released a report titled "Methadone-Associated Mortality, Report of a National Assessment". The consensus report concludes that "although the data remains incomplete, National Assessment meeting participants concurred that methadone tablets or diskettes distributed through channels other than opioid treatment programs most likely are the central factors in methadone-associated mortality."[45]
In 2006, the U.S.
Food and Drug Administration issued a caution about methadone, titled “Methadone Use for Pain Control May Result in Death.”
The FDA also revised the drug's package insert.
The change deleted previous information about the usual adult dosage.
The Charleston Gazette reported, "The old language about the 'usual adult dose' was potentially deadly, according to pain specialists."[46]
Pharmacology
Methadone acts by binding to the µ-opioid receptor, but also has some affinity for the NMDA receptor, an ionotropic glutamate receptor. Methadone is metabolized by CYP3A4, CYP2B6, CYP2D6 and is a substrate for the P-glycoprotein efflux protein in the intestines and brain. The bioavailability and elimination half-life of methadone are subject to substantial interindividual variability. Its main route of administration is oral. Adverse effects include sedation, hypoventilation, constipation and miosis, in addition to tolerance, dependence and withdrawal difficulties. The withdrawal period can be much more prolonged than with other opioids, spanning anywhere from two weeks to several months.
The metabolic half life of methadone differs from its duration of action.
The metabolic half life is 8 to 59 hours (approximately 24 hours for opioid-tolerant people, and 55 hours in opioid-naive people), as opposed to a half life of 1 to 5 hours for morphine.[6] The length of the half life of methadone allows for exhibition of respiratory depressant effects for an extended duration of time in opioid-naive people.[6]
Mechanism of action
Levomethadone (the L enantiomer) is a μ-opioid receptor agonist with higher intrinsic activity than morphine, but lower affinity.[47] Dextromethadone (the S enantiomer) does not affect opioid receptors but binds to the glutamatergic NMDA (N-methyl-D-aspartate) receptor, and acts as an antagonist against glutamate. Methadone has been shown to reduce neuropathic pain in rat models, primarily through NMDA receptor antagonism. Glutamate is the primary excitatory neurotransmitter in the central nervous system. NMDA receptors have a very important role in modulating long-term excitation and memory formation. NMDA antagonists such as dextromethorphan (DXM, a cough suppressant), ketamine (a dissociative anaesthetic), tiletamine (a veterinary anaesthetic) and ibogaine (from the African tree Tabernanthe iboga) are being studied for their role in decreasing the development of tolerance to opioids and as possible for eliminating addiction/tolerance/withdrawal, possibly by disrupting memory circuitry. Acting as an NMDA antagonist may be one mechanism by which methadone decreases craving for opioids and tolerance, and has been proposed as a possible mechanism for its distinguished efficacy regarding the treatment of neuropathic pain. The dextrorotary form (dextromethadone), which acts as an NMDA receptor antagonist and is devoid of opioid activity, has been shown to produce analgesia in experimental models of chronic pain. Methadone also acted as a potent, noncompetitive α3β4 neuronal nicotinic acetylcholine receptor antagonist in rat receptors, expressed in human embryonic kidney cell lines.[48]
| Compound | Affinities(Ki) | Ratios | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MOR | DOR | KOR | SERT | NET | NMDAR | MOR:DOR:KOR | SERT:NET | ||
| Methadone | 1.7 nM | 435 nM | 405 nM | ND | ND | 2,500–8,300 nM | 1:256:238 | ND | [49][50] |
| Dextromethadone | 19.7 nM | 960 nM | 1,370 nM | 992 nM | 12,700 nM | 2,600–7,400 nM | 1:49:70 | 1:13 | [49][50] |
| Levomethadone | 0.945 nM | 371 nM | 1,860 nM | 14.1 nM | 702 nM | 2,800–3,400 nM | 1:393:1968 | 1:50 | [49][50] |
Metabolism
Methadone has a slow metabolism and very high fat solubility, making it longer lasting than morphine-based drugs. Methadone has a typical elimination half-life of 15 to 60 hours with a mean of around 22. However, metabolism rates vary greatly between individuals, up to a factor of 100,[51][52] ranging from as few as 4 hours to as many as 130 hours,[53] or even 190 hours.[54] This variability is apparently due to genetic variability in the production of the associated cytochrome enzymes CYP3A4, CYP2B6 and CYP2D6. Many substances can also induce, inhibit or compete with these enzymes further affecting (sometimes dangerously) methadone half-life. A longer half-life frequently allows for administration only once a day in Opioid detoxification and maintenance programs. People who metabolize methadone rapidly, on the other hand, may require twice daily dosing to obtain sufficient symptom alleviation while avoiding excessive peaks and troughs in their blood concentrations and associated effects.[53] This can also allow lower total doses in some such people. The analgesic activity is shorter than the pharmacological half-life; dosing for pain control usually requires multiple doses per day normally dividing daily dosage for administration at 8 hour intervals.[55]
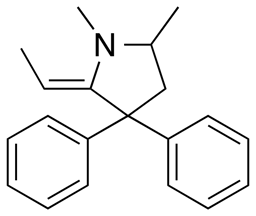
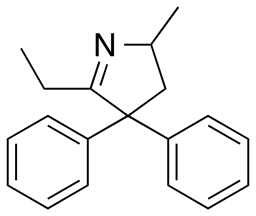
The main metabolic pathway involves N-demethylation by CYP3A4 in the liver and intestine to give 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP).[2][56] This inactive product, as well as the inactive 2-ethyl-5-methyl-3,3- diphenyl-1-pyrroline (EMDP), produced by a second N-demethylation, are detectable in the urine of those taking methadone.
Route of administration
The most common route of administration at a methadone clinic is in a racemic oral solution, though in Germany, only the R enantiomer (the L optical isomer) has traditionally been used, as it is responsible for most of the desired opioid effects.[53] The single-isomer form is becoming less common due to the higher production costs.
Methadone is available in traditional pill, sublingual tablet, and two different formulations designed for the person to drink. Drinkable forms include ready-to-dispense liquid (sold in the United States as Methadose), and "Diskets"(known on the street as "wafers" or "biscuits") which are tablets designed to disperse themselves rapidly in water for oral administration, used in a similar fashion to Alka-Seltzer. The liquid form is the most common as it allows for smaller dose changes. Methadone is almost as effective when administered orally as by injection. Oral medication is usually preferable because it offers safety, simplicity and represents a step away from injection-based drug abuse in those recovering from addiction. U.S. federal regulations require the oral form in addiction treatment programs.[57] Injecting methadone pills can cause collapsed veins, bruising, swelling, and possibly other harmful effects. Methadone pills often contain talc that, when injected, produces a swarm of tiny solid particles in the blood, causing numerous minor blood clots.[58][59] These particles cannot be filtered out before injection, and will accumulate in the body over time, especially in the lungs and eyes, producing various complications such as pulmonary hypertension, an irreversible and progressive disease.[60][61][62] The formulation sold under the brand name Methadose (flavored liquid suspension for oral dosing, commonly used for maintenance purposes) should not be injected either.[63] U.S. federal regulations require the oral form in addiction treatment programs.[57]
Information leaflets included in packs of UK methadone tablets state that the tablets are for oral use only and that use by any other route can cause serious harm.
In addition to this warning, additives have now been included into the tablets formulation to make the use of them by the IV route more difficult.[64]
Chemistry
Detection in biological fluids
Methadone and its major metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), are often measured in urine as part of a drug abuse testing program, in plasma or serum to confirm a diagnosis of poisoning in hospitalized victims, or in whole blood to assist in a forensic investigation of a traffic or other criminal violation or a case of sudden death. Methadone usage history is considered in interpreting the results as a chronic user can develop tolerance to doses that would incapacitate an opioid-naive individual. Chronic users often have high methadone and EDDP baseline values.[65]
History
40 mg of methadone
Methadone was developed in 1937 in Germany by scientists working for I.G. Farbenindustrie AG at the Farbwerke Hoechst who were looking for a synthetic opioid that could be created with readily available precursors, to solve Germany's opium shortage problem.[66][67] On September 11, 1941 Bockmühl and Ehrhart filed an application for a patent for a synthetic substance they called Hoechst 10820 or Polamidon (a name still in regular use in Germany) and whose structure had only slight relation to morphine or the opiate alkaloids. (Bockmühl and Ehrhart, 1949) It was brought to market in 1943 and was widely used by the German army during WWII.[66]
In the 1930s, pethidine (meperidine) went into production in Germany; however, production of methadone, then being developed under the designation Hoechst 10820, was not carried forward because of side effects discovered in the early research.[68] After the war, all German patents, trade names and research records were requisitioned and expropriated by the Allies. The records on the research work of the I.G. Farbenkonzern at the Farbwerke Hoechst were confiscated by the U.S. Department of Commerce Intelligence, investigated by a Technical Industrial Committee of the U.S. Department of State and then brought to the US.[66] The report published by the committee noted that while methadone was potentially addictive, it produced less sedation and respiratory depression than morphine and was thus interesting as a commercial drug.[66]
In the early 1950s, methadone (most times the racemic HCl salts mixture) was also investigated for use as an antitussive.[69]
Isomethadone, noracymethadol, LAAM, and normethadone were first developed in Germany, United Kingdom, Belgium, Austria, Canada, and the United States in the thirty or so years after the 1937 discovery of pethidine, the first synthetic opioid used in medicine. These synthetic opioids have increased length and depth of satiating any opiate cravings and generate very strong analgesic effects due to their long metabolic half-life and strong receptor affinity at the mu opioid receptor sites. Therefore, they impart much of the satiating and anti-addictive effects of methadone by means of suppressing drug cravings.[70]
It was only in 1947 that the drug was given the generic name “methadone” by the Council on Pharmacy and Chemistry of the American Medical Association.
Since the patent rights of the I.G.
Farbenkonzern and Farbwerke Hoechst were no longer protected each pharmaceutical company interested in the formula could buy the rights for the commercial production of methadone for just one dollar (MOLL 1990).
Methadone was introduced into the United States in 1947 by Eli Lilly and Company as an analgesic under the trade name Dolophine.[66]
The trade name Dolophine was created by Eli Lilly after World War II and used in the United States; the claim that Nazi leader Adolf Hitler ordered the manufacture of methadone or that the brand name 'Dolophine' was named after him is an urban legend. "Dolo" stems from the Latin word for pain, dolor, and finis which means "end". Therefore, Dolophine literally means "pain end".[71] The pejorative term "adolphine" (never a widely used name for the drug) appeared in the United States in the early 1970s as a reference to the aforementioned urban myth that the trade name Dolophine was a reference to Adolf Hitler.[72][73]
Society and culture
Brand names
Brand names include Dolophine, Symoron, Amidone, Methadose, Physeptone, Metadon and Heptadon among others.
Cost
In the US, generic methadone tablets are inexpensive, with retail prices ranging from $0.25 to $2.50 per defined daily dose.[74] Brand-name methadone tablets may cost much more.
Methadone maintenance clinics in the US may be covered by private insurances, Medicaid, or Medicare.[75] Medicare covers methadone under the prescription drug benefit, Medicare Part D, when is it is prescribed for pain, but not when it is used for opioid dependence treatment because it cannot be dispensed in a retail pharmacy for this purpose.[76] In California methadone maintenance treatment is covered under the medical benefit. Patients' eligibility for methadone maintenance treatment is also contingent on them being enrolled in substance abuse counseling.[77] The United States Department of Veteran's Affairs (VA) Alcohol and Drug Dependence Rehabilitation Program offers methadone services to eligible veterans enrolled in the VA health care system.[78]
Methadone maintenance treatment (MMT) cost analyses often compare the cost of clinic visits versus the overall societal costs of illicit opioid use.[79][80] A preliminary cost analysis conducted in 2016 by the US Department of Defense determined that methadone treatment, which includes psychosocial and support services, may cost an average of $126.00 per week or $6,552.00 per year.[81]
As of 2015 China had the largest methadone maintenance treatment program with over 250,000 people in over 650 clinics in 27 provinces.[82]
Controversy
Methadone substitution as a treatment of opioid addiction has been criticized in the social sciences for its role in social control of addicts.[83] It is suggested that methadone does not function as much to curb addiction as to redirect it and maintain dependency on authorised channels.
Several authors apply a Foucauldian analysis to the widespread prescription of the drug and use in institutions such as prisons, hospitals and rehabilitation centres.[84] Such critique centers on the notion that substance addiction is reframed with a disease model. Thus methadone, which mimics the effects of opioids and renders the addict compliant, is labeled as a “treatment” and so obscures the disciplinary objectives of “managing undesirables”.[83]
Regulation
Methadone is a Schedule I controlled substance in Canada and Schedule II in the United States, with an ACSCN of 9250 and a 2014 annual aggregate manufacturing quota of 31,875 kilos for sale. Methadone intermediate is also controlled, under ACSCN 9226 also under Schedule II, with a quota of 38,875 kilos. In most countries of the world, methadone is similarly restricted. The salts of methadone in use are the hydrobromide (free base conversion ratio 0.793), hydrochloride (0.894), and HCl monohydrate (0.850).[85] Methadone is also regulated internationally as a Schedule I controlled substance under the United Nations Single Convention on Narcotic Drugs of 1961.[86][87]
In Russia, methadone treatment is illegal. Gennadiy Onishchenko, Chief Sanitary Inspector of Russia, claimed in 2008 that health officials are not convinced of the treatment's efficacy. Instead, doctors encourage immediate cessation of drug use, rather than the gradual process that methadone substitution therapy entails. People are often given sedatives and non-opioid analgesics to cope with withdrawal symptoms.[88]