Studies on Cannabinoids in Cancer
Studies on Cannabinoids in Cancer
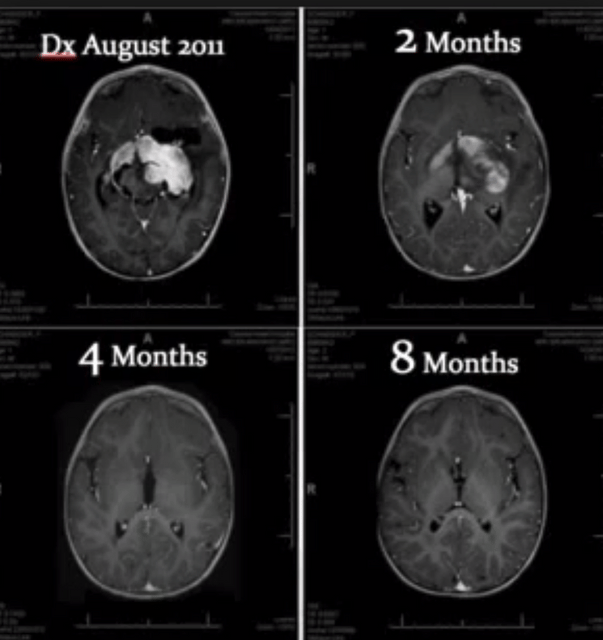
There is a growing amount of evidence supporting the claim that certain compounds found in Cannabis plants may inhibit tumor growth.
Overview
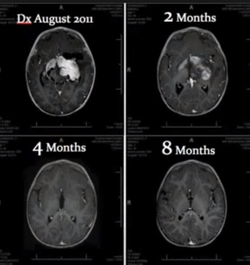
There have been many studies performed to determine the efficacy of cannabinoids at treating cancer tumors.
The studies have mostly been conducted on animals and in vitro, though there are an increasing amount of studies performed on human subjects.
The safest and most effective method of administration is by ingesting concentrated oils orally.
Smoking cannabis releases harmful carcinogens and tar, damaging the lungs.
Topical ointments are used by some to treat melanoma skin cancer or localized cancers.
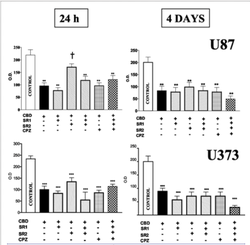
Cannabinoids have been shown to have anti-tumor effects in four key areas:
Antimetastatic :
Cannabinoids have been shown to mitigate the spread of cancerous cells by shutting off the mitochondria in tumor cells.
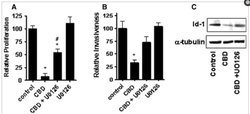
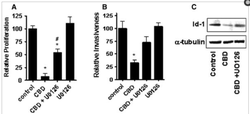
Breast cancer tumors have had their growth inhibited by cannabinoids with higher CBD concentrations.
Cannabinoids have been shown to induce programmed cell death in cancerous cells while leaving healthy cells unharmed.
Synthetic cannabinoids like JWH-133 have also proven to be effective at treating human cancer cells.
Cannibinoids induce Cancer-Cell Apoptosis while Protecting Healthy Cells.
Antiangiogenic :
Prevents the formation of new blood vessels to and from the cancerous tumors.
Certain ratios 1:1 THC to CBD are more effective, for other tumors, higher CBD is more effective.
For instance, Glioma brain caner requires higher concentrations of the lipid THC, due to the fatty blood-brain barrier.
Breast Cancer may have more CBD receptors.
Decarboxylation- removing the acid group from THC-A, isolating THC can be achieved by heating up the cannabis oils.
Potentially each patient, based on their type of cancer, will require a unique ratio of cannabinoids.
Antiproliferative:
Cannabinoids may prevent the spread of cancer cells to nearby organs.
Apoptotic:
Cannabinoids have been shown to induce programmed cell death.
This is particularly important since cannabinoids are able to destroy cancer cells, instead of simply reducing or preventing the spread.