Aneurysm
Aneurysm

| aneurysm | |
|---|---|
| Other names | Aneurism |
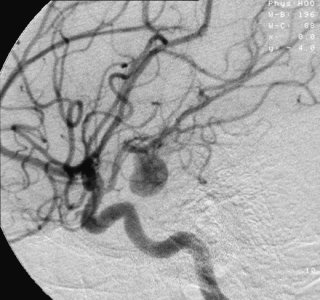
| Angiography of an aneurysm in a brain artery.The aneurysm is the large bulge in the center of the image. | |
| Specialty | Vascular surgery |
An aneurysm is an outward bulging, likened to a bubble or ballon, caused by a localized, abnormal, weak spot on a blood vessel wall.[1] Aneurysms are a result of a weakened blood vessel wall, and may be a result of a hereditary condition or an acquired disease. Aneurysms can also be a nidus (starting point) for clot formation (thrombosis) and embolization. The word is from Greek: ἀνεύρυσμα, aneurysma, "dilation", from ἀνευρύνειν, aneurynein, "to dilate". As an aneurysm increases in size, the risk of rupture increases,[2] leading to uncontrolled bleeding. Although they may occur in any blood vessel, particularly lethal examples include aneurysms of the Circle of Willis in the brain, aortic aneurysms affecting the thoracic aorta, and abdominal aortic aneurysms. Aneurysms can arise in the heart itself following a heart attack, including both ventricular and atrial septal aneurysms.
| aneurysm | |
|---|---|
| Other names | Aneurism |
| Angiography of an aneurysm in a brain artery.The aneurysm is the large bulge in the center of the image. | |
| Specialty | Vascular surgery |
Classification
Aneurysms are classified by type, morphology, or location.
True and false aneurysms
A true aneurysm is one that involves all three layers of the wall of an artery (intima, media and adventitia). True aneurysms include atherosclerotic, syphilitic, and congenital aneurysms, as well as ventricular aneurysms that follow transmural myocardial infarctions (aneurysms that involve all layers of the attenuated wall of the heart are also considered true aneurysms).[3]
A false aneurysm, or pseudoaneurysm, is a collection of blood leaking completely out of an artery or vein, but confined next to the vessel by the surrounding tissue. This blood-filled cavity will eventually either thrombose (clot) enough to seal the leak, or rupture out of the surrounding tissue.[3]
Morphology
Saccular aneurysms are spherical in shape and involve only a portion of the vessel wall; they vary in size from 5 to 20 cm (2.0 to 7.9 in) in diameter, and are often filled, either partially or fully, by a thrombus.[3]
Fusiform aneurysms ("spindle-shaped" aneurysms) are variable in both their diameter and length; their diameters can extend up to 20 cm (7.9 in).
They often involve large portions of the ascending and transverse aortic arch, the abdominal aorta, or less frequently the iliac arteries.[3]
Location
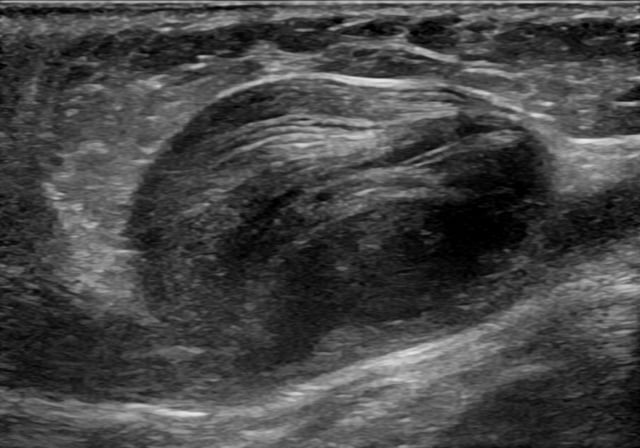
Ultrasonography of an aneurysm of the great saphenous vein due to venous valve insufficiency.
Aneurysms can also be classified by their location:
Arterial and venous, with arterial being more common.
The heart, including coronary artery aneurysms, ventricular aneurysms, aneurysm of sinus of Valsalva, and aneurysms following cardiac surgery.
The aorta, namely aortic aneurysms including thoracic aortic aneurysms and abdominal aortic aneurysms.[8]
The brain, including cerebral aneurysms, berry aneurysms, and Charcot–Bouchard aneurysms.
The legs, including the popliteal arteries.
The kidney, including renal artery aneurysm and intraparechymal aneurysms.[9]
Capillaries, specifically capillary aneurysms.
Cerebral aneurysms, also known as intracranial or brain aneurysms, occur most commonly in the anterior cerebral artery, which is part of the circle of Willis. This can cause severe strokes leading to death. The next most common sites of cerebral aneurysm occurrence are in the internal carotid artery.[10]
Size
Abdominal aortic aneurysms are commonly divided according to their size and symptomatology.
An aneurysm is usually defined as an outer aortic diameter over 3 cm (normal diameter of the aorta is around 2 cm),[13] or more than 50% of normal diameter that of a healthy individual of the same sex and age.[8][14] If the outer diameter exceeds 5.5 cm, the aneurysm is considered to be large.[12]
The common iliac artery is classified as:[15]
| Normal | Diameter ≤12 mm |
| Ectatic | Diameter 12 to 18 mm |
| Aneurysm | Diameter ≥18 mm |
Signs and symptoms
Aneurysm presentation may range from life-threatening complications of hypovolemic shock to being found incidentally on X-ray.[16] Symptoms will differ by the site of the aneurysm and can include:
Cerebral aneurysm
Symptoms can occur when the aneurysm pushes on a structure in the brain.
Symptoms will depend on whether an aneurysm has ruptured or not.
There may be no symptoms present at all until the aneurysm ruptures.[17] For an aneurysm that has not ruptured the following symptoms can occur:
Fatigue
Loss of perception
Loss of balance
Speech problems
Double vision
For a ruptured aneurysm, symptoms of a subarachnoid hemorrhage may present:
Severe headaches
Loss of vision
Double vision
Neck pain or stiffness
Pain above or behind the eyes
Abdominal aneurysm
Illustration depicting location of abdominal aneurysm

3D model of Aortic aneurism
Abdominal aortic aneurysm involves a regional dilation of the aorta and is diagnosed using ultrasonography, computed tomography, or magnetic resonance imaging. A segment of the aorta that is found to be greater than 50% larger than that of a healthy individual of the same sex and age is considered aneurysmal.[8] Abdominal aneurysms are usually asymptomatic but in rare cases can cause lower back pain or lower limb ischemia.
Renal (kidney) aneurysm
Flank pain and tenderness
Hypertension
Haematuria
Signs of hypovolemic shock
Risk factors
Risk factors for an aneurysm include diabetes, obesity, hypertension, tobacco use, alcoholism, high cholesterol, copper deficiency, increasing age, and tertiary syphilis infection.[16]
Specific infective causes associated with aneurysm include:
Advanced syphilis infection resulting in syphilitic aortitis and an aortic aneurysm
Tuberculosis, causing Rasmussen's aneurysms
Brain infections, causing infectious intracranial aneurysms
A minority of aneurysms are associated with genetic factors.
Examples include:
Berry aneurysms of the anterior communicating artery of the circle of Willis, associated with autosomal dominant polycystic kidney disease[18]
Familial thoracic aortic aneurysms
Cirsoid aneurysms, secondary to congenital arteriovenous malformations
Pathophysiology
Aneurysms form for a variety of interacting reasons.
Multiple factors, including factors affecting a blood vessel wall and the blood through the vessel, contribute.
Atherosclerosis. A variety of different factors, including atherosclerosis, may contribute to weakening of a blood vessel wall. The repeated trauma of blood flowing through the vessel may contribute to degeneration of the vessel wall. Hypertensive injury may compound this degeneration and accelerate the expansion of the aneurysm. As the aneurysm expands, the wall tension increases.[19]
The pressure of blood within the expanding aneurysm may also injure the blood vessels supplying the artery itself, further weakening the vessel wall. Without treatment, these aneurysms will ultimately progress and rupture.[20]
Infection. A mycotic aneurysm is an aneurysm that results from an infectious process that involves the arterial wall.[21] A person with a mycotic aneurysm has a bacterial infection in the wall of an artery, resulting in the formation of an aneurysm. The most common locations include arteries in the abdomen, thigh, neck, and arm. A mycotic aneurysm can result in sepsis, or life-threatening bleeding if the aneurysm ruptures. Less than 3% of abdominal aortic aneurysms are mycotic aneurysms.[22]
Syphilis. The third stage of syphilis also manifests as aneurysm of the aorta, which is due to loss of the vasa vasorum in the tunica adventitia.[23]
Copper deficiency. A minority of aneurysms are caused by copper deficiency, which results in a decreased activity of the lysyl oxidase enzyme, affecting elastin, a key component in vessel walls.[24][25][26] Copper deficiency results in vessel wall thinning,[27] and thus has been noted as a cause of death in copper-deficient humans,[28] chickens and turkeys[29]
Mechanics
Aneurysmal blood vessels are prone to rupture under normal blood pressure and flow due to their special mechanical properties that make them weaker.
To better understand this phenomenon, we can first look at healthy arterial vessels which exhibit a J-shaped stress-strain curve with high strength and high toughness (for a biomaterial in vivo).[30] Unlike crystalline materials whose linear elastic region follows Hooke’s Law under uniaxial loading, many biomaterials exhibit a J-shaped stress-strain curve which is non-linear and concave up.[30] The blood vessel can be under large strain, or the amount of stretch the blood vessel can undergo, for a range of low applied stress before fracture, as shown by the lower part of the curve. The area under the curve up to a given strain is much lower than that for the equivalent Hookean curve, which is correlated to toughness. Toughness is defined as the amount of energy per unit volume a material can absorb before rupturing. Because the amount of energy release is proportional to the amount of crack propagation, the blood vessel wall can withstand pressure and is “tough.” Thus, healthy blood vessels with the mechanical properties of the J-shaped stress-strain curve have greater stability against aneurysms than materials with linear elasticity.
Blood vessels with aneurysms, on the other hand, are under the influence of an S-shaped stress-strain curve.
As a visual aid, aneurysms can be understood as a long, cylindrical balloon.
Because it’s a tight balloon under pressure, it can pop at any time a stress beyond a certain force threshold is applied.
In the same vein, an unhealthy blood vessel has elastic instabilities that lead to rupture.[30] Initially, for a given radius and pressure, stiffness of the material increases linearly.
At a certain point, the stiffness of the arterial wall starts to decrease with increasing load.
At higher strain values, the area under the curve increases, thus increasing the impact on the material that would promote crack propagation.
The differences in the mechanical properties of the aneurysmal blood vessels and the healthy blood vessels stem from the compositional differences of the vessels.
Compared to normal aortas, aneurysmal aortas have a much higher volume fraction of collagen and ground substance (54.8% vs. 95.6%) and a much lower volume fraction of elastin (22.7% vs. 2.4%) and smooth muscles (22.6% vs. 2.2%), which contribute to higher initial stiffness.[31] It was also found that the ultimate tensile strength, or the strength to withstand rupture, of aneurysmal vessel wall is 50% lower than that of normal aortas.[32] The wall strength of ruptured aneurysmal aortic wall was also found to be 54.2 N/cm2, which is much lower than that of a repaired aorta wall, 82.3 N/cm2.[32] Due to the change in composition of the arterial wall, aneurysms overall have much lower strength to resist rupture. Predicting the risk of rupture is difficult due to the regional anisotropy the hardened blood vessels exhibit, meaning that the stress and strength values vary depending on the region and the direction of the vessel they are measured along.[33]
Diagnosis
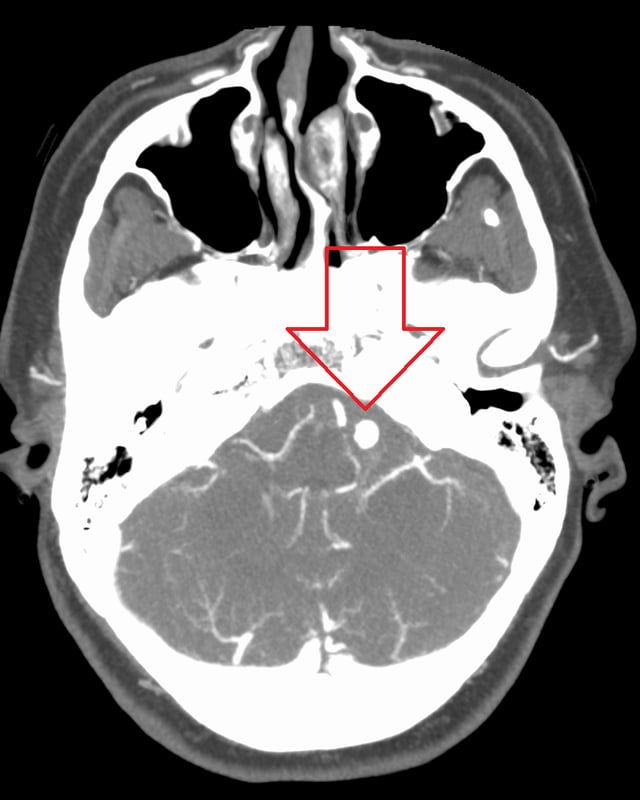
Ruptured 7mm left vertebral artery aneurysm resulting in a subarachnoid hemorrhage as seen on a CT scan with contrast
Diagnosis of a ruptured cerebral aneurysm is commonly made by finding signs of subarachnoid hemorrhage on a computed tomography (CT) scan. If the CT scan is negative but a ruptured aneurysm is still suspected based on clinical findings, a lumbar puncture can be performed to detect blood in the cerebrospinal fluid. Computed tomography angiography (CTA) is an alternative to traditional angiography and can be performed without the need for arterial catheterization. This test combines a regular CT scan with a contrast dye injected into a vein. Once the dye is injected into a vein, it travels to the cerebral arteries, and images are created using a CT scan. These images show exactly how blood flows into the brain arteries.
Treatment
Historically, the treatment of arterial aneurysms has been limited to either surgical intervention, or watchful waiting in combination with control of blood pressure. At least, in case of Abdominal Aortic Aneurysm (AAA) the decision does not come without a significant risk and cost, hence, there is a great interest in identifying more advanced decision making approaches that are not solely based on the AAA diameter, but involve other geometrical and mechanical nuances such as local thickness and wall stress.[8] In recent years, endovascular or minimally invasive techniques have been developed for many types of aneurysms. Aneurysm clips are used for surgical procedure i.e. clipping of aneurysms.[34]
Intracranial aneurysms
There are currently two treatment options for brain aneurysms: surgical clipping or endovascular coiling. There is currently debate in the medical literature about which treatment is most appropriate given particular situations.[35]
Surgical clipping was introduced by Walter Dandy of the Johns Hopkins Hospital in 1937. It consists of a craniotomy to expose the aneurysm and closing the base or neck of the aneurysm with a clip. The surgical technique has been modified and improved over the years.
Endovascular coiling was introduced by Italian neurosurgeon Guido Guglielmi at UCLA in 1989. It consists of passing a catheter into the femoral artery in the groin, through the aorta, into the brain arteries, and finally into the aneurysm itself. Platinum coils initiate a clotting reaction within the aneurysm that, if successful, fills the aneurysm dome and prevents its rupture.[36] Flow diverter can be used but not without complications sometimes.[37]
Aortic and peripheral aneurysms
Endovascular stent and endovascular coil
For aneurysms in the aorta, arms, legs, or head, the weakened section of the vessel may be replaced by a bypass graft that is sutured at the vascular stumps.
Instead of sewing, the graft tube ends, made rigid and expandable by nitinol wireframe, can be easily inserted in its reduced diameter into the vascular stumps and then expanded up to the most appropriate diameter and permanently fixed there by external ligature.[38][39] New devices were recently developed to substitute the external ligature by expandable ring allowing use in acute ascending aorta dissection, providing airtight (i.e. not dependent on the coagulation integrity), easy and quick anastomosis extended to the arch concavity[38][41][38] Less invasive endovascular techniques allow covered metallic stent grafts to be inserted through the arteries of the leg and deployed across the aneurysm.
Renal aneurysms
Renal aneurysms are very rare consisting of only 0.1–0.09%[43] while rupture is even more rare.[43][44] Conservative treatment with control of concomitant hypertension being the primary option with aneurysms smaller than 3 cm. If symptoms occur, or enlargement of the aneurysm, then endovascular or open repair should be considered.[45] Pregnant women (due to high rupture risk of up to 80%) should be treated surgically.[46]
Epidemiology
Incidence rates of cranial aneurysms are estimated at between 0.4% and 3.6%.
Those without risk factors have expected prevalence of 2–3%.[10] In adults, females are more likely to have aneurysms. They are most prevalent in people ages 35 – 60, but can occur in children as well. Aneurysms are rare in children with a reported prevalence of.5% to 4.6%. The most common incidence are among 50-year-olds, and there are typically no warning signs. Most aneurysms develop after the age of 40.
Pediatric aneurysms
Risk factors
Incidence rates are two to three times higher in males, while there are more large and giant aneurysms and fewer multiple aneurysms.[10] Intracranial hemorrhages are 1.6 times more likely to be due to aneurysms than cerebral arteriovenous malformations in whites, but four times less in certain Asian populations.[10]
Most patients, particularly infants, present with subarachnoid hemorrhage and corresponding headaches or neurological deficits.
The mortality rate for pediatric aneurysms is lower than in adults.[10]
Computerized Investigation of Aneurysm Biomechanics
In additional to the biomechanics of aortic wall given that shear stress due to the pulsatile blood flow plays an important role in the disease progression of an aneurysm.
High shear stress could lead to an overexpression of CTGF released by platelets and CTGF overexpression would lead to aortic smooth muscle cell apoptosis, which is the signature of media layer degradation in ATAA.
Finite element simulations have focused on the investigation of aneurysm wall stress in patient-specific modeling using data either from computed tomography (CT) imaging or MRI.
Studies focused on the analysis of the aortic wall stress with the use of anisotropic hyperelastic arterial wall modeling, geometrical correction for zero blood pressure, aortic root displacement, and aneurysm expansion prediction.
The accurate hemodynamic analysis in the cardiovascular system is heavily depended on the appropriate interaction modelling between blood and blood vessels; therefore, accurate models for.blood rheology and vessel structures are necessary to account for blood flow distributions and aortic wall stresses.
Although debates are ongoing regarding the effectiveness of different computational approaches between computational fluid dynamics (CFD) and FSI methods for the investigations of aortic biomechanics, the motions of blood vessel induced under physiological pulsatile blood pressure by FSI approach would have different flow distributions than predicted by CFD approaches.
The prediction from CFD would capture the effects of different non-Newtonian models applied to patient-specific geometry were investigated and it was concluded that the Newtonian model underestimates WSS prediction.
Given the importance in WSS, the PSI approach that couples CPD and structural mechanics for modelling blood vessel expansion (Windkessel effect) should be used toward accurate hemodynamic predictions.
Both PSI-CPD comparison studies by Reymond et al. and Crosetto et al. concluded that there was a WSS overestimation from the simulations without the inclusion of the aortic wall.
There were multiple PSI studies conducted for modelling cardiovascular system in the past decade.
Each of them had a slightly different approach in hemorheological and structural modelling.
Earlier PSI studies focused on the assessment of multilayer aortic wall biomechanics.
Gao et al., constructed idealized 3D Newtonian models for the hemodynamics comparison between non-aneurysm and aneurysm model with aortic wall properties assumed as three isotropic linear elastic layers (intima, media, and adventitia).
Similarly, Khanafer and Berguer's three-layered isotropic linear elastic aorta model provided insights into peak wall stress in the media layer.
The PSI method has also been used recently in the modelling in ATAA geometrical characteristics, ATAA with BAV and TAV, abdominal aortic aneurysm growth evolution, and local stiffening.
While the studies utilized the advanced material model for the aortic wall (isotropic hyperelastic and anisotropic hyperelastic models), the blood was assumed to be a Newtonian fluid.
As mentioned earlier, WSS prediction is one of the most important biomechanics predictors in ATAA, and FSI model with Newtonian fluid would result in a WSS underestimation.
Interestingly, as all the results implied a geometrical dependence in hemodynamic distributions and helical flow development, the quantitative relationship between geometry and hemodynamics is to be further developed for better model predictions and correlations such that the analysis can be translated toward to clinical practice.
Nevertheless, for an FSI modelling, the boundary velocity conditions (3D MRI, 1D MRI, fully developed, and plug flow) prescribed in the model would also have a significant influence in time-averaged WSS distributions and oscillatory shear index, which would affect the predicted outcome.
The hyperelastic models for characterizing the aortic wall are used to determine the biomechanical response under physiological loading conditions.
Tan et al. investigated the mechanics and hemodynamics of TAA using the single layer isotropic hyperelastic Mooney-Rivlin model, with material constants taken from the experimental work on AAA.
The use of laminar-turbulent transition model concluded a 13% lower time-averaged WSS and significantly higher turbulence intensity in the FSI model than the rigid wall model.
On the other hand, Raghavan and Vorp conducted another study on the rupture potential of AAA with freshly excised tissue.
They concluding that AAA wall stress would only vary 4% if the material parameters used from Mooney-Rivlin constitutive equation were within 95% confidence intervals.
This suggests that the use of mean value from sample population might be sufficient for patient-specific modeling.
For advanced numerical simulation, Grytsan et al. have modified the anisotropic arterial wall model and accounted for AAA remodeling under the additional consideration of elastin degradation and adaptation of collagen fibres.
The model considered the hemodynamic changes with the progression of AAA enlargement but without the connection between WSS and AAA remodeling.
The time-averaged WSS was not meaningfully influenced by the arterial wall motion due to pulsatile blood flow unless the investigation of instantaneous WSS was considered.
Since the magnitude of WSS would affect endothelial cell alignment and potentially cause blood cell damage, a coupled fluid and structure study on WSS is of considerable value given that the local blood recirculation or unbalanced homeostasis could also lead to aneurysm rupture.
Notable cases
Lucille Ball, who died from an aortic rupture in the abdominal area days after having undergone apparently successful heart surgery for a dissecting aortic aneurysm[48][49][50]
Laura Branigan, who died of a cerebral aneurysm
David Cone, who suffered from an aneurysm and missed most of the 1996 baseball season
John Olerud, suffered an aneurysm in 1989 and forced to wear batting helmet on field all of his career since then
Albert Einstein, who died from a repaired aortic aneurysm[51]
Thomas Mikal Ford, who died from a ruptured aneurysm in his abdomen. He was 52.
Charles de Gaulle, who died from an aneurysm within his neck[52]
Richard Holbrooke, who died from a thoracic aortic aneurysm[53]
Stuart Sutcliffe, who died from an aneurysm in his brain's right hemisphere[54]
John Ritter, died September 11, 2003 of a misdiagnosed thoracic aortic dissection (aortic aneurysm).[55][56]
Geoffrey Thompson, who died of a brain aneurysm at his daughter's wedding, hosted at his theme park, Blackpool Pleasure Beach.
Edwin Rosario died of an aneurysm in 1997.