Mercury(II) oxide
_oxide-vIfNf3FWtXdEFDSU5UtNrA4uCWgqKw)
Mercury(II) oxide
_oxide-vIfNf3FWtXdEFDSU5UtNrA4uCWgqKw)
 | |
| Names | |
|---|---|
| IUPAC name Mercury(II) oxide | |
| Other names Mercuric oxide Montroydite Red mercury | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.040.580 [21] |
| KEGG |
|
PubChemCID |
|
| RTECS number | OW8750000 |
| UN number | 1641 |
CompTox Dashboard(EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
| HgO | |
| Molar mass | 216.591 g·mol−1 |
| Appearance | Yellow or red solid |
| Odor | odorless |
| Density | 11.14 g/cm3 |
| Melting point | 500 °C (932 °F; 773 K) (decomposes) |
Solubility in water | 0.0053 g/100 mL (25 °C) 0.0395 g/100 mL (100 °C) |
| Solubility | insoluble in alcohol, ether, acetone, ammonia |
| Band gap | 2.2 eV[1] |
Magnetic susceptibility (χ) | −44.0·10−6cm3/mol |
| 2.5 (550 nm)[1] | |
| Structure | |
Coordination geometry | orthorhombic |
| Thermochemistry | |
Std molar
entropy(S | 70 J·mol−1·K−1[2] |
Std enthalpy of
formation(ΔfH⦵298) | −90 kJ·mol−1[2] |
| Hazards | |
| Main hazards | Highly toxic |
| Safety data sheet | ICSC 0981 [25] |
| GHS pictograms |    |
| NFPA 704 |  0 4 2 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50(median dose) | 18 mg/kg (oral, rat)[3] |
| Related compounds | |
Other anions | Mercury sulfide Mercury selenide Mercury telluride |
Other cations | Zinc oxide Cadmium oxide |
Related compounds | Mercury(I) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, has a formula of HgO. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found.
 | |
| Names | |
|---|---|
| IUPAC name Mercury(II) oxide | |
| Other names Mercuric oxide Montroydite Red mercury | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.040.580 [21] |
| KEGG |
|
PubChemCID |
|
| RTECS number | OW8750000 |
| UN number | 1641 |
CompTox Dashboard(EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
| HgO | |
| Molar mass | 216.591 g·mol−1 |
| Appearance | Yellow or red solid |
| Odor | odorless |
| Density | 11.14 g/cm3 |
| Melting point | 500 °C (932 °F; 773 K) (decomposes) |
Solubility in water | 0.0053 g/100 mL (25 °C) 0.0395 g/100 mL (100 °C) |
| Solubility | insoluble in alcohol, ether, acetone, ammonia |
| Band gap | 2.2 eV[1] |
Magnetic susceptibility (χ) | −44.0·10−6cm3/mol |
| 2.5 (550 nm)[1] | |
| Structure | |
Coordination geometry | orthorhombic |
| Thermochemistry | |
Std molar
entropy(S | 70 J·mol−1·K−1[2] |
Std enthalpy of
formation(ΔfH⦵298) | −90 kJ·mol−1[2] |
| Hazards | |
| Main hazards | Highly toxic |
| Safety data sheet | ICSC 0981 [25] |
| GHS pictograms |    |
| NFPA 704 |  0 4 2 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50(median dose) | 18 mg/kg (oral, rat)[3] |
| Related compounds | |
Other anions | Mercury sulfide Mercury selenide Mercury telluride |
Other cations | Zinc oxide Cadmium oxide |
Related compounds | Mercury(I) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
Synthesis
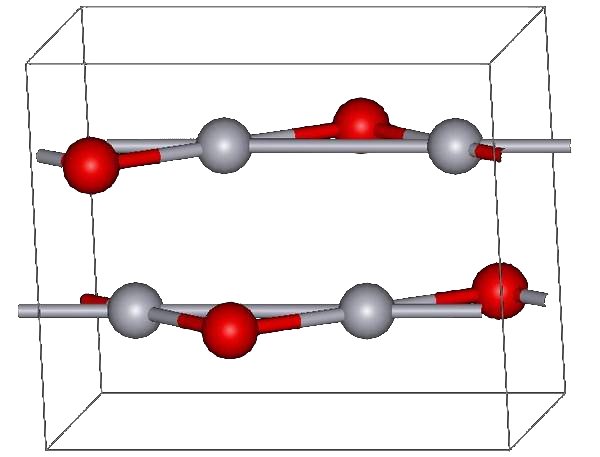
Montroydite structure (red atoms are oxygens)
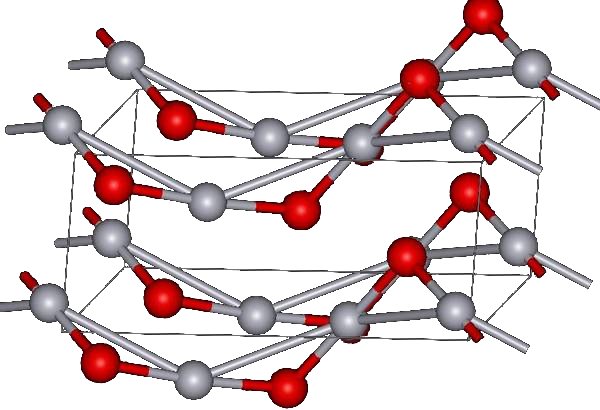
Cinnabar structure
The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis of Hg(NO3)2.[5] The yellow form can be obtained by precipitation of aqueous Hg2+ with alkali.[5] The difference in color is due to particle size, both forms have the same structure consisting of near linear O-Hg-O units linked in zigzag chains with an Hg-O-Hg angle of 108°.[5]
Structure
Under atmospheric pressure mercuric oxide has two crystalline forms: one is called montroydite (orthorhombic, 2/m 2/m 2/m, Pnma), and the second is analogous to the sulfide mineral cinnabar (hexagonal, hP6, P3221); both are characterized by Hg-O chains.[6] At pressures above 10 GPa both structures convert to a tetragonal form.[1]
Uses
HgO is sometimes used in the production of mercury as it decomposes quite easily. When it decomposes, oxygen gas is generated.
It is also used as a material for cathodes for mercury batteries.[7]
Health issues

The label on an HgO powder bottle.
Mercury oxide is a highly toxic substance which can be absorbed into the body by inhalation of its aerosol, through the skin and by ingestion. The substance is irritating to the eyes, the skin and the respiratory tract and may have effects on the kidneys, resulting in kidney impairment. In the food chain important to humans, bioaccumulation takes place, specifically in aquatic organisms. The substance is banned as a pesticide in the EU.[8]
Evaporation at 20 °C is negligible. HgO decomposes on exposure to light or on heating above 500 °C. Heating produces highly toxic mercury fumes and oxygen, which increases the fire hazard. Mercury(II) oxide reacts violently with reducing agents, chlorine, hydrogen peroxide, magnesium (when heated), disulfur dichloride and hydrogen trisulfide. Shock-sensitive compounds are formed with metals and elements such as sulfur and phosphorus.[9]