Friedel–Crafts reaction

Friedel–Crafts reaction

| Friedel-Crafts reaction | |
|---|---|
| Named after | Charles Friedel James Crafts |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000369 [46] |
| Friedel-Crafts alkylation | |
| Named after | Charles Friedel James Crafts |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | friedel-crafts-alkylation [47] |
| RSC ontology ID | RXNO:0000046 [48] |
| Friedel-Crafts acylation | |
| Named after | Charles Friedel James Crafts |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | friedel-crafts-acylation [49] |
| RSC ontology ID | RXNO:0000045 [50] |
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring.[1] Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.[2][3][4][5]
| Friedel-Crafts reaction | |
|---|---|
| Named after | Charles Friedel James Crafts |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000369 [46] |
| Friedel-Crafts alkylation | |
| Named after | Charles Friedel James Crafts |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | friedel-crafts-alkylation [47] |
| RSC ontology ID | RXNO:0000046 [48] |
| Friedel-Crafts acylation | |
| Named after | Charles Friedel James Crafts |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | friedel-crafts-acylation [49] |
| RSC ontology ID | RXNO:0000045 [50] |
Friedel–Crafts alkylation
For primary (and possibly secondary) alkyl halides, a carbocation-like complex with the Lewis acid, [R(+)---(X---MXn)(–)] is more likely to be involved, rather than a free carbocation.
This reaction suffers from the disadvantage that the product is more nucleophilic than the reactant because alkyl groups are activators for the Friedel–Crafts reaction. Consequently, overalkylation can occur. Steric hindrance can be exploited to limit the number of alkylations, as in the t-butylation of 1,4-dimethoxybenzene.[8]
Furthermore, the reaction is only useful for primary alkyl halides in an intramolecular sense when a 5- or 6-membered ring is formed. For the intermolecular case, the reaction is limited to tertiary alkylating agents, some secondary alkylating agents (ones for which carbocation rearrangement is degenerate), or alkylating agents that yield stabilized carbocations (e.g., benzylic or allylic ones). In the case of primary alkyl halides, the carbocation-like complex (R(+)---X---Al(-)Cl3) will undergo a carbocation rearrangement reaction to give almost exclusively the rearranged product derived from a secondary or tertiary carbocation.[7]
- H2C=C(CH3)CH2Cl + C6H6→ C6H5C(CH3)2CH2Cl
In one study the electrophile is a bromonium ion derived from an alkene and NBS:[10]
In this reaction samarium(III) triflate is believed to activate the NBS halogen donor in halonium ion formation.
Friedel–Crafts dealkylation
Friedel–Crafts alkylation has been hypothesized to be reversible. In a retro-Friedel–Crafts reaction or Friedel–Crafts dealkylation, alkyl groups are removed in the presence of protons or other Lewis acid.
For example, in a multiple addition of ethyl bromide to benzene, ortho and para substitution is expected after the first monosubstitution step because an alkyl group is an activating group. However, the actual reaction product is 1,3,5-triethylbenzene with all alkyl groups as a meta substituent.[11] Thermodynamic reaction control makes sure that thermodynamically favored meta substitution with steric hindrance minimized takes prevalence over less favorable ortho and para substitution by chemical equilibration. The ultimate reaction product is thus the result of a series of alkylations and dealkylations.[12]
Friedel–Crafts acylation
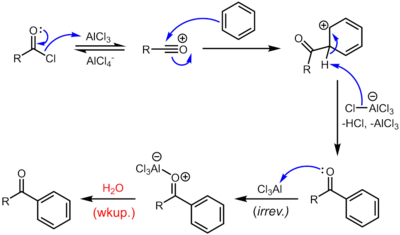
Friedel–Crafts acylation involves the acylation of aromatic rings. Typical acylating agents are acyl chlorides. Typical Lewis acid catalysts are acids and aluminium trichloride. However, because the product ketone forms a rather stable complex with Lewis acids such as AlCl3, a stoichiometric amount or more of the "catalyst" must generally be employed, unlike the case of the Friedel–Crafts alkylation, in which the catalyst is constantly regenerated. Friedel–Crafts acylation is also possible with acid anhydrides.[13] Reaction conditions are similar to the Friedel–Crafts alkylation. This reaction has several advantages over the alkylation reaction. Due to the electron-withdrawing effect of the carbonyl group, the ketone product is always less reactive than the original molecule, so multiple acylations do not occur. Also, there are no carbocation rearrangements, as the acylium ion is stabilized by a resonance structure in which the positive charge is on the oxygen.
The viability of the Friedel–Crafts acylation depends on the stability of the acyl chloride reagent. Formyl chloride, for example, is too unstable to be isolated. Thus, synthesis of benzaldehyde through the Friedel–Crafts pathway requires that formyl chloride be synthesized in situ. This is accomplished by the Gattermann-Koch reaction, accomplished by treating benzene with carbon monoxide and hydrogen chloride under high pressure, catalyzed by a mixture of aluminium chloride and cuprous chloride.
Reaction mechanism
The reaction proceeds through generation of an acylium center. The reaction is completed by deprotonation of the arenium ion by AlCl4−, regenerating the AlCl3 catalyst. Nevertheless, in contrast to the alkylation reaction, the formed ketone is a moderate nucleophile, which forms a complex with aluminum trichloride. The formation of this complex is typically irreversible under reaction conditions. Thus, a stochiometric quantity of AlCl3 is needed. The complex is destroyed upon aqueous workup to give the desired ketone. For example, the classical synthesis of deoxybenzoin calls for 1.1 equivalents of AlCl3 with respect to the limiting reagent, phenylacetyl chloride.[14]
If desired, the resulting ketone can be subsequently reduced to the corresponding alkane substituent by either Wolff–Kishner reduction or Clemmensen reduction. The net result is the same as the Friedel–Crafts alkylation except that rearrangement is not possible.[15]
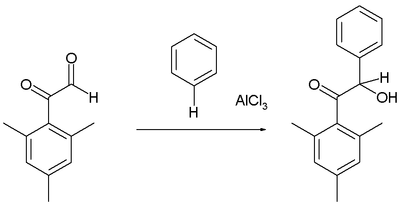
Friedel–Crafts hydroxyalkylation
Arenes react with certain aldehydes and ketones to form the hydroxyalkylated products, for example in the reaction of the mesityl derivative of glyoxal with benzene:[16]
As usual, the aldehyde group is more reactive electrophile than the phenone.
Scope and variations
This reaction is related to several classic named reactions:
The acylated reaction product can be converted into the alkylated product via a Clemmensen reduction.[17][18][19]
The Gattermann–Koch reaction can be used to synthesize benzaldehyde from benzene.[20]
The Gatterman reaction describes arene reactions with hydrocyanic acid.[21]
The Houben–Hoesch reaction describes arene reactions with nitriles.[22][23]
A reaction modification with an aromatic phenyl ester as a reactant is called the Fries rearrangement.
In the Scholl reaction two arenes couple directly (sometimes called Friedel–Crafts arylation).[24][25]
In the Zincke–Suhl reaction p-cresol is alkylated to a cyclohexadienone with tetrachloromethane.[26]
In the Blanc chloromethylation a chloromethyl group is added to an arene with formaldehyde, hydrochloric acid and zinc chloride.[27][28]
The Bogert–Cook synthesis (1933) involves the dehydration and isomerization of 1-β-phenylethylcyclohexanol to the octahydro derivative of phenanthrene[29]
[[INLINE_IMAGE|//upload.wikimedia.org/wikipedia/commons/thumb/0/0d/Bogert-Cook_Synthesis.png/500px-Bogert-Cook_Synthesis.png|//upload.wikimedia.org/wikipedia/commons/0/0d/Bogert-Cook_Synthesis.png 1.5x|Bogert-Cook Synthesis.png|h130|w500]]
The Darzens–Nenitzescu synthesis of ketones (1910, 1936) involves the acylation of cyclohexene with acetyl chloride to methylcyclohexenylketone.
In the related Nenitzescu reductive acylation (1936) a saturated hydrocarbon is added making it a reductive acylation to methylcyclohexylketone
The Nencki reaction (1881) is the ring acetylation of phenols with acids in the presence of zinc chloride.[30]
In a green chemistry variation aluminium chloride is replaced by graphite in an alkylation of p-xylene with 2-bromobutane. This variation will not work with primary halides from which less carbocation involvement is inferred.[31]
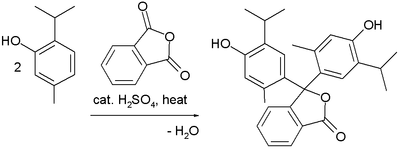
Dyes
Friedel–Crafts reactions have been used in the synthesis of several triarylmethane and xanthene dyes.[32] Examples are the synthesis of thymolphthalein (a pH indicator) from two equivalents of thymol and phthalic anhydride:
A reaction of phthalic anhydride with resorcinol in the presence of zinc chloride gives the fluorophore fluorescein. Replacing resorcinol by N,N-diethylaminophenol in this reaction gives rhodamine B:
Haworth reactions
In a related reaction, phenanthrene is synthesized from naphthalene and succinic anhydride in a series of steps which begin with FC acylation.
Friedel–Crafts test for aromatic hydrocarbons
Reaction of chloroform with aromatic compounds using an aluminium chloride catalyst gives triarylmethanes, which are often brightly colored, as is the case in triarylmethane dyes. This is a bench test for aromatic compounds.[35]
See also
Ethylene oxide
Friedel family, a rich lineage of French scientists
Hydrodealkylation
Transalkylation