Dulong–Petit law
Dulong–Petit law

The Dulong–Petit law, a thermodynamic law proposed in 1819 by French physicists Pierre Louis Dulong and Alexis Thérèse Petit, states the classical expression for the molar specific heat capacity of certain chemical elements. Experimentally the two scientists had found that the heat capacity per weight (the mass-specific heat capacity) for a number of elements was close to a constant value, after it had been multiplied by a number representing the presumed relative atomic weight of the element. These atomic weights had shortly before been suggested by John Dalton and modified by Jacob Berzelius.
In modern terms, Dulong and Petit found that the heat capacity of a mole of many solid elements is about 3R, where R is the modern constant called the universal gas constant. Dulong and Petit were unaware of the relationship with R, since this constant had not yet been defined from the later kinetic theory of gases. The value of 3R is about 25 joules per kelvin, and Dulong and Petit essentially found that this was the heat capacity of certain solid elements per mole of atoms they contained.
The modern theory of the heat capacity of solids states that it is due to lattice vibrations in the solid and was first derived in crude form from this assumption by Albert Einstein in 1907. The Einstein solid model thus gave for the first time a reason why the Dulong–Petit law should be stated in terms of the classical heat capacities for gases.
Equivalent forms of statement of the law
An equivalent statement of the Dulong–Petit law in modern terms is that, regardless of the nature of the substance, the specific heat capacity c of a solid element (measured in joule per kelvin per kilogram) is equal to 3R/M, where R is the gas constant (measured in joule per kelvin per mole) and M is the molar mass (measured in kilogram per mole). Thus, the heat capacity per mole of many elements is 3R.
The initial form of the Dulong–Petit law was:

where K is a constant which we know today is about 3R.
In modern terms the mass m of the sample divided by molar mass M gives the number of moles n.

Therefore, using uppercase C for the full heat capacity (in joule per kelvin), we have:

or
 .
.Therefore, the heat capacity of most solid crystalline substances is 3R per mole of substance.
Dulong and Petit did not state their law in terms of the gas constant R (which was not then known). Instead, they measured the values of heat capacities (per weight) of substances and found them smaller for substances of greater atomic weight as inferred by Dalton and other early atomists. Dulong and Petit then found that when multiplied by these atomic weights, the value for the heat capacity per mole was nearly constant, and equal to a value which was later recognized to be 3R.
In other modern terminology, the dimensionless heat capacity (C/NR) is equal to 3.
The law can also be written as a function of the total number of atoms N in the sample:
 ,
,where kB is Boltzmann constant.
Application limits
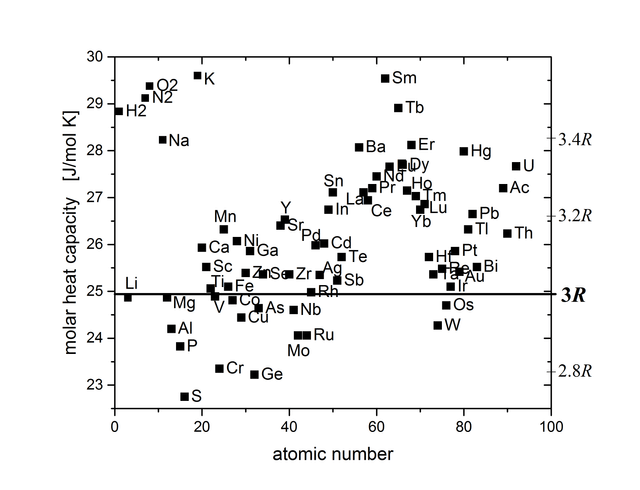
The molar heat capacity plotted of most elements at 25°C plotted as a function of atomic number. The value of bromine is for the gaseous state. For iodine, a value for the gas and one for the solid is shown.
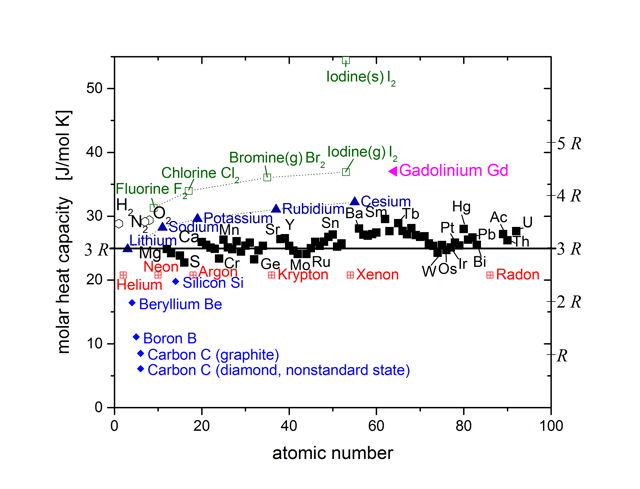
Despite its simplicity, Dulong–Petit law offers fairly good prediction for the heat capacity of many elementary solids with relatively simple crystal structure at high temperatures. This agreement is because in the classical statistical theory of Ludwig Boltzmann, the heat capacity of solids approaches a maximum of 3R per mole of atoms because full vibrational-mode degrees of freedom amount to 3 degrees of freedom per atom, each corresponding to a quadratic kinetic energy term and a quadratic potential energy term. By the equipartition theorem, the average of each quadratic term is 1⁄2kBT, or 1⁄2RT per mole (see derivation below). Multiplied by 3 degrees of freedom and the two terms per degree of freedom, this amounts to 3R per mole heat capacity.
The Dulong–Petit law fails at room temperatures for light atoms bonded strongly to each other, such as in metallic beryllium and in carbon as diamond. Here, it predicts higher heat capacities than are actually found, with the difference due to higher-energy vibrational modes not being populated at room temperatures in these substances.
In the very low (cryogenic) temperature region, where the quantum mechanical nature of energy storage in all solids manifests itself with larger and larger effect, the law fails for all substances. For crystals under such conditions, the Debye model, an extension of the Einstein theory that accounts for statistical distributions in atomic vibration when there are lower amounts of energy to distribute, works well.
Derivation for an Einstein solid
A system of vibrations in a crystalline solid lattice can be modelled as an Einstein solid, i.e.by considering N quantum harmonic oscillator potentials along each degree of freedom. Then, the free energy of the system can be written as[1]

where the index α sums over all the degrees of freedom. In the 1907 Einstein model (as opposed to the later Debye model) we consider only the high-energy limit:

Then

and we have

Define geometric mean frequency by

where g measures the total number of spatial degrees of freedom of the system.
Thus we have

Using energy

we have

This gives heat capacity at constant volume

which is independent of the temperature.
For another more precise derivation, see Debye model.
See also
Stefan–Boltzmann law
Kopp–Neumann law