alpha-Pyrrolidinopentiophenone
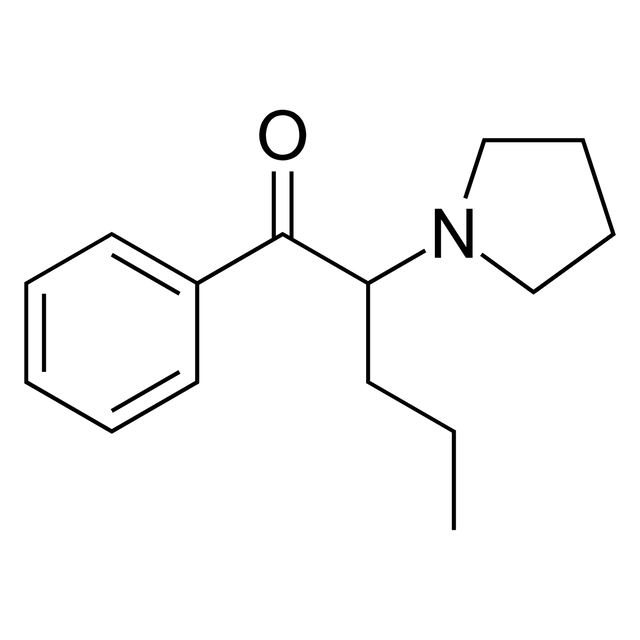
alpha-Pyrrolidinopentiophenone

 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, intranasal, vaporization, intravenous, rectal, sublingual |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChemCID |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| CompTox Dashboard(EPA) |
|
| Chemical and physical data | |
| Formula | C15H21NO |
| Molar mass | 231.339 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
α-Pyrrolidinopentiophenone (also known as α-pyrrolidinovalerophenone, α-PVP, O-2387, β-keto-prolintane, prolintanone, or desmethylpyrovalerone) is a synthetic stimulant of the cathinone class developed in the 1960s[2] that has been sold as a designer drug.[3] Colloquially, it is sometimes called flakka.[1] α-PVP is chemically related to pyrovalerone and is the ketone analog of prolintane.[6]
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral, intranasal, vaporization, intravenous, rectal, sublingual |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChemCID |
|
| ChemSpider |
|
| UNII |
|
| ChEMBL |
|
| CompTox Dashboard(EPA) |
|
| Chemical and physical data | |
| Formula | C15H21NO |
| Molar mass | 231.339 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
Adverse effects
α-PVP, like other psychostimulants, can cause hyperstimulation, paranoia, and hallucinations.[7] α-PVP has been reported to be the cause, or a significant contributory cause of death in suicides and overdoses caused by combinations of drugs.[8][9][10][11] α-PVP has also been linked to at least one death with pulmonary edema and moderately advanced atherosclerotic coronary disease when it was combined with pentedrone.[12]
Pharmacology
Chemistry
α-PVP gives no reaction with the Marquis reagent. It gives a grey/black reaction with the Mecke reagent.[19]
Detection in body fluids
α-PVP may be quantified in blood, plasma or urine by liquid chromatography-mass spectrometry to confirm a diagnosis of poisoning in hospitalized patients or to provide evidence in a medicolegal death investigation. Blood or plasma α-PVP concentrations are expected to be in a range of 10–50 μg/L in persons using the drug recreationally, >100 μg/L in intoxicated patients and >300 μg/L in victims of acute overdosage.[20][21]
Society and culture
Legal status
As of October 2015, α-PVP is a controlled substance in China.[24]
In Australia Alpha-PVP is a Schedule 9 prohibited substance under the Poisons Standard (July 2016).[26] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[26] The drug was explicitly made illegal in New South Wales after it was illegally marketed with the imprimatur of erroneous legal advice that it was not encompassed by analog provisions of the relevant act. It is encompassed by those provisions, and therefore has been illegal for many years in New South Wales. The legislative action followed the death of two individuals from using it; one jumping off a balcony, another having a heart attack after a state of delirium.[27][28]
Economics
α-PVP is sometimes the active ingredient in recreational drugs sold as "bath salts".[27] It may also be distinguished from "bath salts" and sold under a different name: "flakka", a name used in Florida, or "gravel" in other parts of the U.S. It is reportedly available as cheaply as US$5 per dose.[30] A laboratory for one county in Florida reported a steady rise in α-PVP detections in seized drugs from none in January–February 2014 to 84 in September 2014.[31]
See also
α-Pyrrolidinohexiophenone (α-PHP)
α-Pyrrolidinopentiothiophenone (α-PVT)
4'-Methoxy-α-Pyrrolidinopentiophenone
Naphyrone (O-2482)
Pentedrone
Pentylone
Prolintane
Pyrovalerone (O-2371)