1,4,6-Androstatriene-3,17-dione
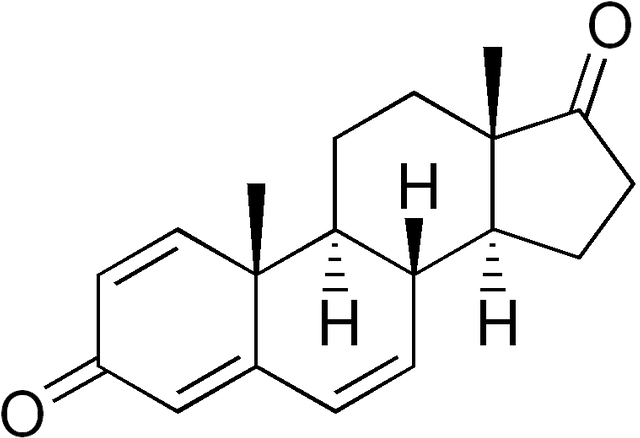
1,4,6-Androstatriene-3,17-dione

| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 48 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChemCID |
|
| ChemSpider |
|
| ChEBI |
|
| Chemical and physical data | |
| Formula | C19H22O2 |
| Molar mass | 282 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
| (verify) [16] | |
ATD was present in some over-the-counter bodybuilding supplements until 2009 as well as Topical ATD solutions that work transdermally. The product was developed and commercialized in the dietary supplement market place by industry journeyman, Bruce Kneller who holds a United States Patent for use of the compound and related compounds (#7,939,517) and Gaspari Nutrition. ATD has many names in sports supplements including: 1,4,6 etiollochan-dione, 3, 17-keto-etiochol-triene, androst-1,4,6-triene-3,17-dione and many others. These all refer to CAS# 633-35-2.
ATD may cause a positive test for the anabolic steroid boldenone, of which it is a possible metabolite and production contaminant and is also prohibited in amateur and professional sports which forbids aromatase inhibitors.[3]
A related agent is exemestane (Aromasin).
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 48 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChemCID |
|
| ChemSpider |
|
| ChEBI |
|
| Chemical and physical data | |
| Formula | C19H22O2 |
| Molar mass | 282 g·mol−1 |
| 3D model (JSmol) |
|
SMILES
| |
InChI
| |
| (verify) [16] | |